Neural Conduction and Neurotransmitters
Additional Videos and Readings
Video – Author/Source: Khan Academy; Neuron Action Potential description
Neuron action potentials play a crucial role in transmitting information over long distances. The all-or-none property of action potentials and the speed-enhancing function of myelin sheaths are key aspects of this process. The distinction between graded potentials and action potentials is also vital, each contributing uniquely to neural signaling.
Video- Author/Source: Khan Academy; Types of neurotransmitters and Types of neurotransmitter receptors
This video gives an overview of neurotransmitter receptors in neurons. Learn how they can be excitatory or inhibitory, and how neurotransmitters can bind to multiple types of receptors. Discover the role of ionotropic and metabotropic receptors, and how they influence neuron behavior, from rapid effects to long-lasting changes.
Steve Ramirez and Xu Liu discuss their research on memory at MIT, including an overview of where and how memory formation occurs in the brain, and how they experimented with test mice to create and trigger specific memories with a light-sensitive switch. Further experiments showed that memories can also be edited by activating specific memories while creating new associations. The talk ends with a call to action to think about the ethical implications of this work and where neuroscience research can and should go from here.
Chapter 4: Electrical Properties of Neurons
One of the interesting properties of neurons is that their cell membranes are electroactive, meaning that they are sensitive to electrical charge. Neurons are capable of changing the electrical potential of their membranes, which makes them a very dynamic population of cells. These changes, which can happen on the scale of milliseconds, provide a rapid method for one part of a neuron to send signals over long distances – hence, the analogy of electrical wires running overhead. In this chapter, we will go into depth describing the molecular and cellular components that contribute to the electrical properties of a neuron, both at rest and during an action potential.
4.1 Ion channels
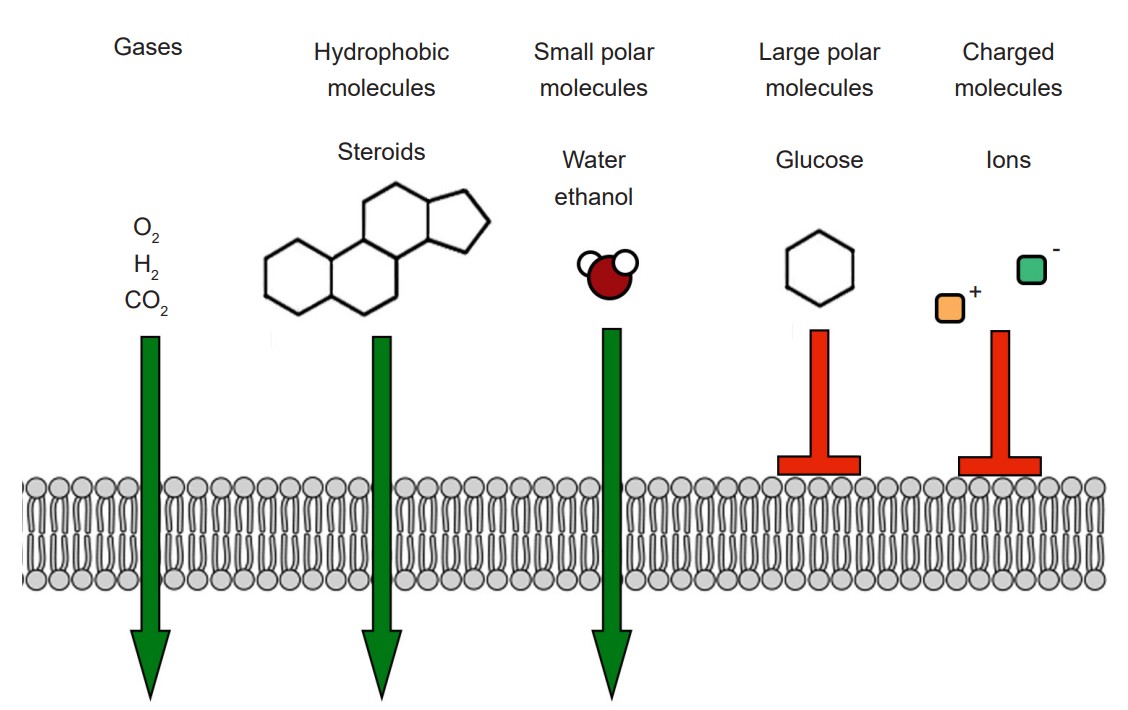
The cell membrane that separates the inside of the cell from the outside is a very effective boundary. It is described as being selectively permeable, which means that some molecules are able to travel across the membrane easily, other molecules have an intermediate ability to cross, and other molecules are completely incapable of passing. Generally, gases and molecules of water are able to pass through the cell membrane easily. Large molecules like glucose, and charged molecules like ions or amino acids, are unable to pass across the membrane.
Most cells of the body, including neurons, have specialized transmembrane proteins embedded in the cell membrane. These transmembrane proteins are huge protein complexes that span the entirety of the membrane, with an outer side and an inner side. In the middle of the protein is a pore, which is essentially a “tunnel” that allows molecules and ions to pass across the cell membrane. These proteins are called ion channels. These channels are passive since they do not use any cellular energy to move ions. Rather, they simply provide easy passage for ions. It may be useful to think of an ion channel as a “cellular door.”
One important feature of ion channels is their ability to distinguish ions based on their chemical properties. For example, some channels are selective for Na+, while preventing the passage of all other ions. Each ion channel has special molecular characteristics that allow certain types of ions to pass through the pore while excluding other ions. Some features that allow for distinction between ions include:
1. Pore size. The molecular shape of an ion channel can exclude larger diameter ions if it is small enough, restricting the ability for the large ion to enter the pore – in the same way that a ping pong ball cannot pass through a narrow diameter of a straw.
2. Electrical charge. Ion channels can also prevent certain ions from passing through based on the electrical charge. The inside of the pore is lined with charged amino acids, and their charge allows only certain types of ions to pass. For example, sodium channels have several negatively-charged glutamate residues inside the pore. Because of this, negatively-charged ions like chloride will be repelled by the negative charges, but the positively-charged sodium ions will be attracted to the inside.
3. Hydration shell. Selectivity based on charge and small size is a simple task, but how can it be possible for an ion channel to allow larger ions to pass while excluding smaller ions? This is possible because it is energetically favorable to allow an ion that has been stabilized sufficiently by the properties of the amino acid residues lining the pore.
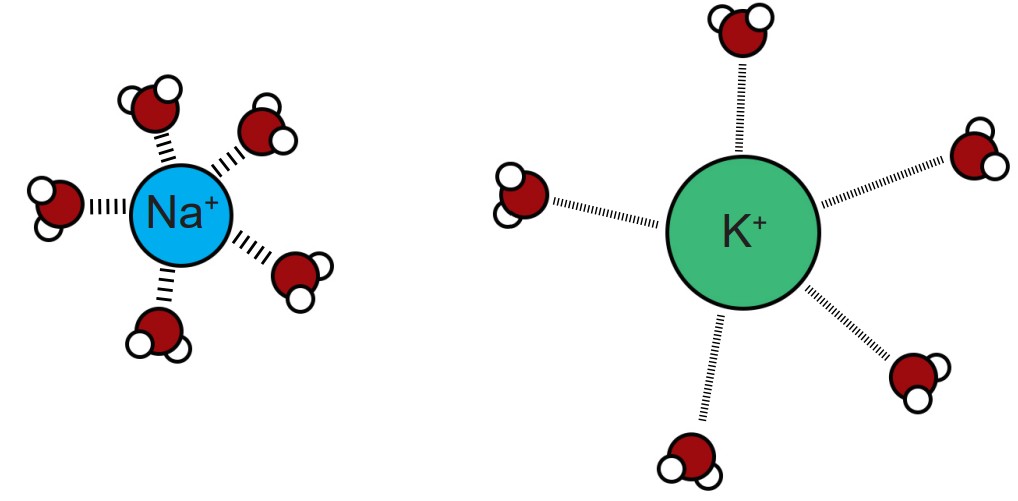
This process is related to the observation that a shell of water molecules surrounds each charged ion in solution. To pass through the pore, water molecules must be separated from the ion. The potassium ion has diffuse hydration shell, and so it is easier to separate water from a potassium ion compared to a sodium ion. The passage of potassium becomes more energetically favorable compared to passing a sodium ion, causing these pores to be selective.
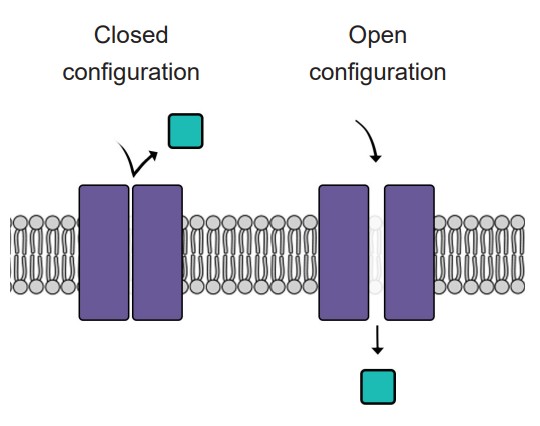
Like doors, different types of ion channels open and close under different conditions. We can categorize ion channels into four major classes based on their opening and closing conditions.
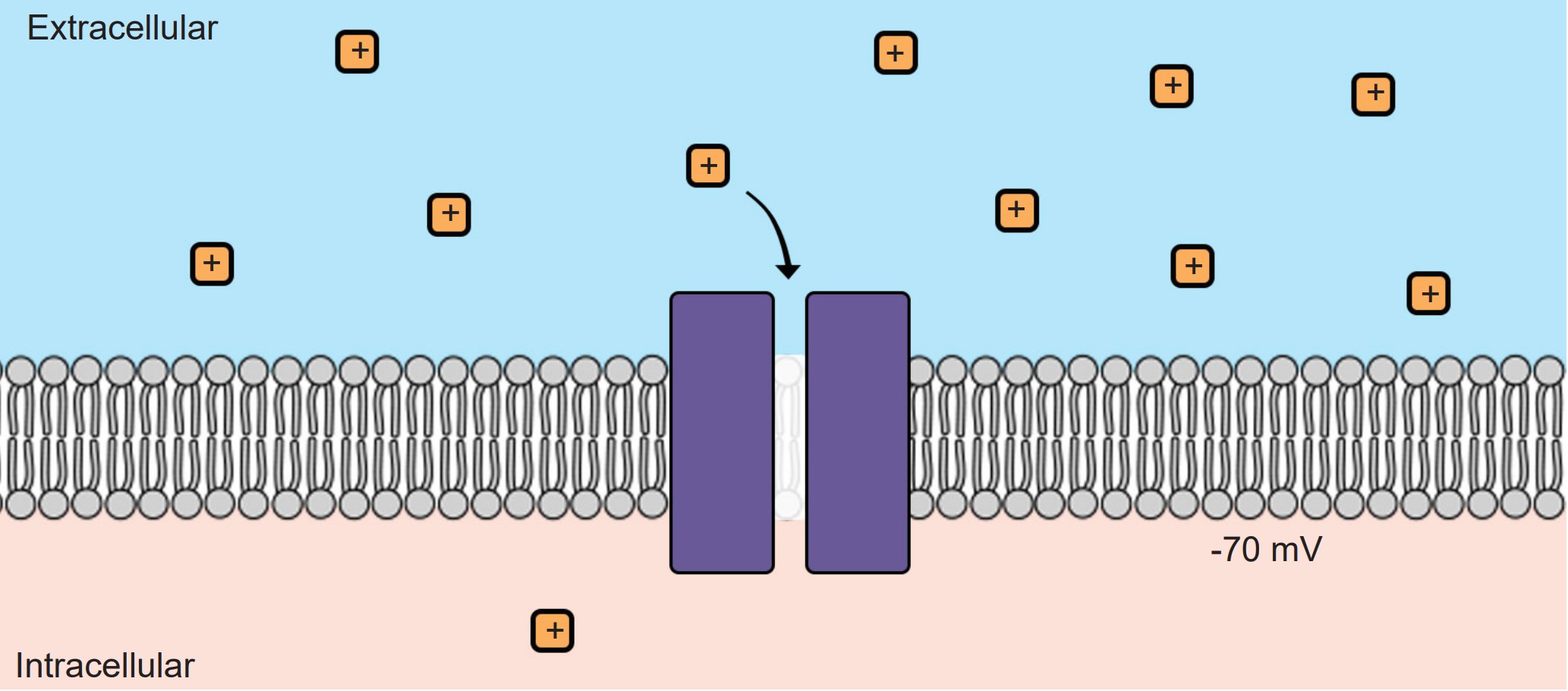
1. Leak channels are persistently open. You can think of these leak channels as revolving doors that are never locked. Neurons usually have several leak channels, such as a leak channel for potassium (K+) and a leak channel for chloride (Cl- ) ions. These two ions are at equilibrium, flowing back and forth across the cell membrane, and they help contribute to the properties of the neuron at rest.
2. Voltage-gated ion channels are sensitive to the electrical potential of the surrounding membrane. Their ability to open and close depends on the electrical charge of the membrane. Many of them, such as voltage-gated sodium (Na+) channels, remain closed at negative potentials (resting condition) and only open at positive potentials (action potential).
3. Ligand-gated ion channels open in response to binding certain molecules (ligands), such as neurotransmitters. These types of ion channels are also called ionotropic receptors, and these will be described in much more depth in chapter 5.
4. The fourth class of ion channels is a catch-all category that includes a wide variety of channels that are used by the sensory systems. They open and close in response to unique stimuli depending on what they are able to sense. For example, some open and close depending when they are moved physically, such as a distortion or stretch. We have these in our ears (hair cells, chapter 8.1), in our skin (Pacinian corpuscles, chapter 8.3) and in our muscles (Golgi tendon organ, chapter 10). Photoreceptors in our eyes have ion channels that close in response to being hit by photons of light, and this activity is necessary for us to be able to see in both brightly and dimly lit environments.
4.2 The electrochemical gradient
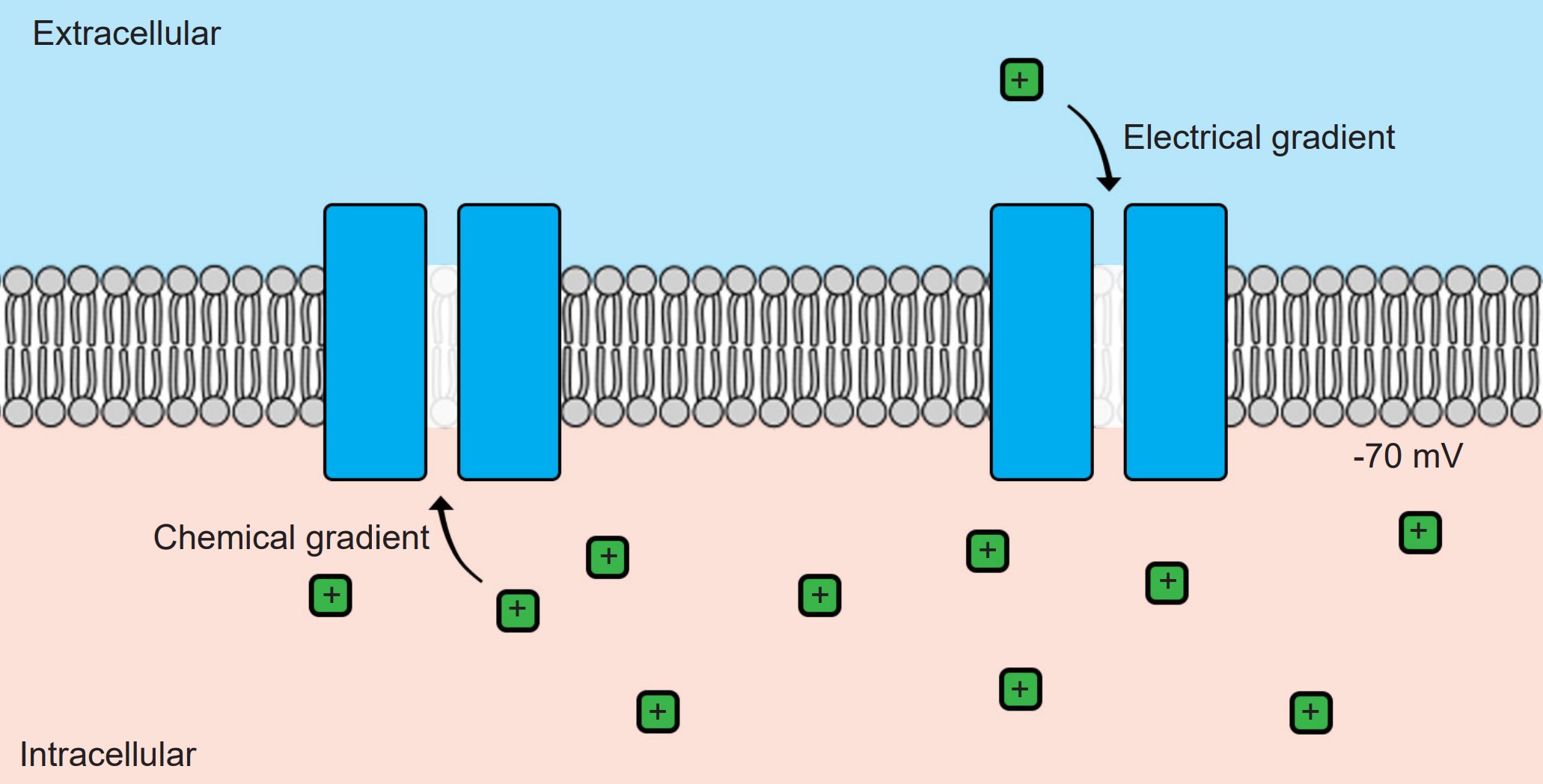
Without ion channels, ions do not cross the cell membrane and therefore stay either outside or inside the cell. But when an ion channel is present and open, ions can move across the cell membrane, depending on the specific conditions inside and outside the neuron. There are two different forces that act on ions once an ion channel is open: the electrical gradient and the chemical gradient.
1. Electrical gradient
The electrical gradient refers to the electrical forces acting on charged molecules, “pulling” opposite charges together while also “pushing” like charges away from one another – just like the polarity of magnets.
At rest, the interior of the cell has a negative charge, about -70 mV. Because of these negative charges inside the cell, the electrical gradient causes positively charged ions to be attracted to the inside of the cell. At the same time, negatively charged ions will be repelled out of the cell.
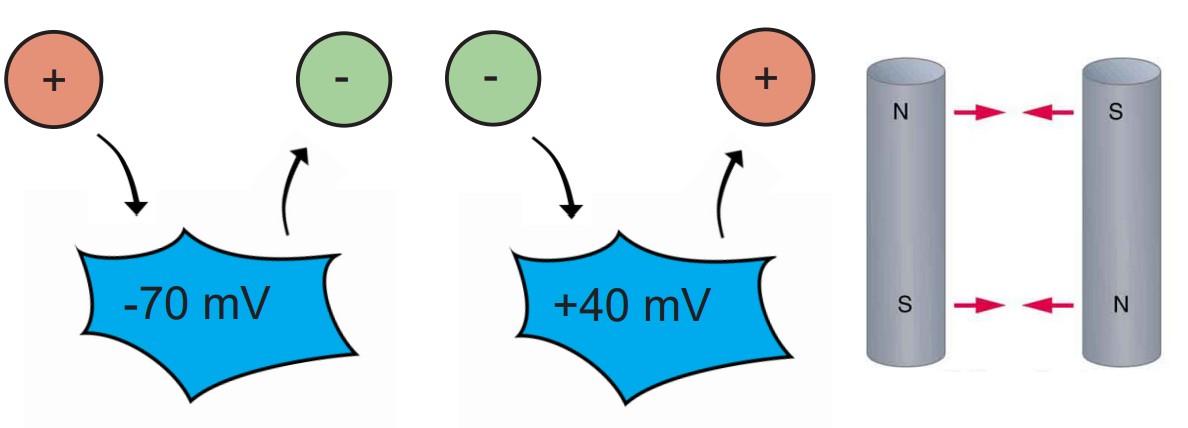
The voltage of the neuron (Vm) can deviate significantly from -70 mV, reaching potentials as high as +40 mV. When the Vm changes, the force exerted by the electrical gradient changes as well. If the cell now has a positive potential instead of a negative potential, movement of charged ions will change as well. In this case, the electrical gradient now attracts negatively charged ions into the cell while pushing positively charged ions out of the cell.
2. Chemical gradient
The chemical gradient refers to the natural process by which a high concentration of a substance, given enough time, will eventually diffuse to a lower concentration and settle evenly over the space.
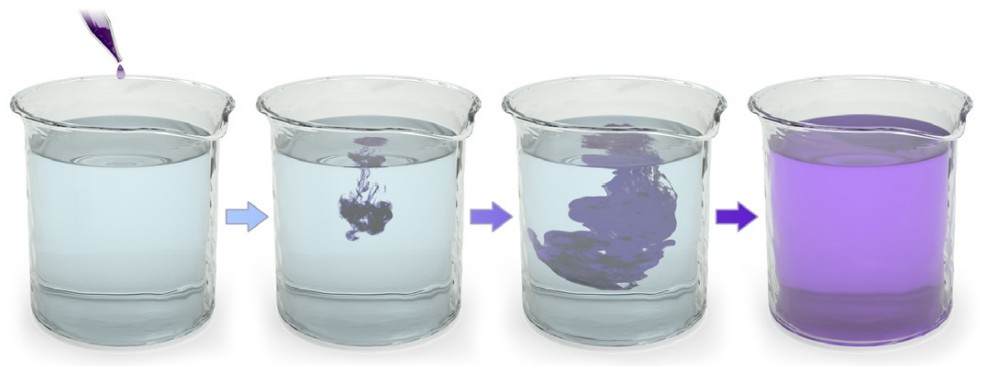
You can think of the chemical gradient as similar to the way people behave when they exit a full elevator. People don’t want to be so close to each other inside a cramped space. Once the doors open, people scatter quickly, going to where they can have more personal space. They move from an area of high concentration to low concentration. Molecules behave the same way.
Unlike the electrical gradient, the chemical gradient is unaffected by biological movement of ions. In other words, the distribution of ions across the membrane does not change significantly. The number of ions that flow across the cell membrane is so minute that the relative distribution of ions across the cell membrane essentially does not change.
These two forces are collectively called the electrochemical gradient. In order to accurately predict the forces acting on a given ion, you need to know 1) the charge of the ion and 2) the relative concentrations of ions across the membrane.
Take a look at the following two examples:
1. Sodium (Na+) movement across the membrane
Consider the positively-charged ion, sodium (Na+). Because it has a positive charge, it is electrically attracted to the inside of the cell, which has a negative charge at rest. Therefore, when a sodium-permeable ion channel opens and Na+ is now able to pass across the cell membrane, the electrical gradient causes the sodium to move into the cell.
We also know that the concentration of sodium outside the cell (written as [Na+]o , where the brackets indicate concentration and the “o” refers to “outside”) is high compared to the concentration of sodium inside the cell (written as [Na+]i ) by roughly an order of magnitude. Therefore, when that sodium-permeable ion channel opens, Na+ will move from the area of high concentration to low concentration, causing Na+ to move into the cell.
Given that both the electrical and chemical gradients are in favor of moving Na+ into the cell, opening a sodium channel causes a net flow of Na+ ions into the cell.
2. Potassium (K+) movement across the membrane
Just like with sodium, we can use our knowledge of potassium’s charge and the distribution of K+ ions across the membrane in order to predict the forces acting on K+ ions when a potassium channel is open.
We know that the inside of the cell is negative and K+ has a positive charge, so the electrical gradient causes K+ to be attracted into the cell. We also know that the inside of the cell has a relatively high concentration of K+ ions compared to the outside of the cell; therefore the chemical gradient pushes K+ out of the cell.
Since the ion concentrations across the membrane do not change significantly, but the membrane voltage can, you could imagine there exists some Vm where the flow of ions moving in are exactly equal to the flow of ions moving out. In this case, the two forces acting on the ion oppose one another perfectly, resulting in a dynamic equilibrium (This process is called “dynamic” because there is a constant movement of ions going in and going out, and yet it is at an “equilibrium” because there is no net movement of charge: the charge moving in is balanced by the charge moving out.) This exact value of the Vm is called the equilibrium potential for the ion, which is also abbreviated Ex where x is the ion of interest. The equilibrium potential differs for each ion, and is described in Table 4.1.
| Ion | Abbreviation | Concentration outside [X]o | Concentration inside [X]i |
| Sodium | Na+ | 140 mM | 15 mM |
| Potassium | K+ | 5 mM | 150 mM |
| Chloride | Cl– | 120 mM | 10 mM |
| Calcium | Ca2+ | 1 mM | 100 nM = 0.1 µM |
| Magnesium | Mg2+ | 2 mM | 0.5 mM |
| Miscellaneous anions | A– | 20 mM | 100 mM |
4.3 Calculating Ex and the Nernst equation
Even though the equilibrium potential (Ex) for each ion can be determined experimentally, it is also possible to calculate Ex using a mathematical equation called the Nernst equation. By understanding the Nernst equation, you can predict the direction that ions will move when an ion channel opens given various conditions. The Nernst equation is as follows:
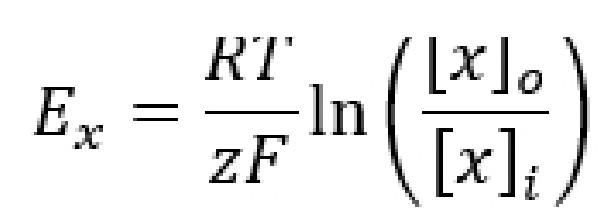
Ex is the equilibrium potential. As a potential, it has units in volts, usually measured in millivolts. When a cell’s membrane potential is exactly at this Ex, the ion is at equilibrium: ion movement into the cell is matched by ion movement out of the cell. Ex is sometimes also called the reversal potential, because once the cell membrane crosses from this potential, the net movement of the ions reverses directions. This value is also called the Nernst potential in honor of Walther Nernst, the Nobel prize-winning German chemist who first developed the formula to describe electrochemical reactions.
R is the ideal gas constant. It has a value of 8.314 J / K * mol. The R term is included in the equation because it is a way to convert between the number of molecules and the energy that these molecules exert.
T is the temperature in Kelvin (to convert from Celsius into Kelvin, add 273 to the Celsius value. C + 273 = K). Usually, room temperature is around 293 K, and biological temperatures are closer to 310 K. This term is included in the equation since all biological processes are temperature dependent, especially those at the level of molecular machinery.
z is the electrical charge of the ion. For sodium and potassium, z is +1, for chloride or monovalent anions z is -1, and for divalent cations like calcium and magnesium, z is +2. This term is essential since the Nernst potential is used to predict the direction of ion movement based on the charge of the ion. This value represents the influence exerted by the electrical gradient.
F is the Faraday constant which has a value of 96,485 Coulombs / mol.
[x]o and [x]i represent the concentration of ion x outside the cell and inside the cell, respectively. Their units are generally in mM, but these units cancel out in the equation. These values represent the forces acting on the ions by the chemical gradient.
The Nernst equation has many complex constants and terms, so neuroscientists often use the back-of-the-envelope equation as a shortcut for quickly calculating the equilibrium potential. This shortcut equation condenses down the R and F constants, turns the natural log into a base 10 logarithm, and assumes the calculations are done at physiological temperature. The shortcut formula can be written as:
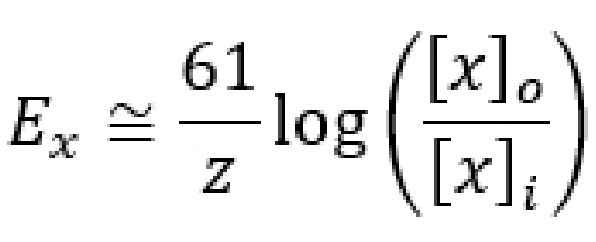
The Nernst equation is able to calculate the reversal potential for individual ions assuming that the appropriate ion channels are open. However, in neurons, not all ion channels are opened or closed at the same time. In a neuron at rest, usually Na+ does not enter into the cells since sodium channels are closed. K+ ions , on the other hand, are often moving through leak channels all the time. Chloride channels are generally open as well, but they pass less current at rest than potassium channels do.
A formula called the Goldman-HodgkinKatz equation (GHK equation) combines the Nernst potentials of three relevant ions (Na+, K+, and Cl- ) into a single equation that, when evaluated, gives us the value of the membrane potential Vm.
The GHK equation is written as:
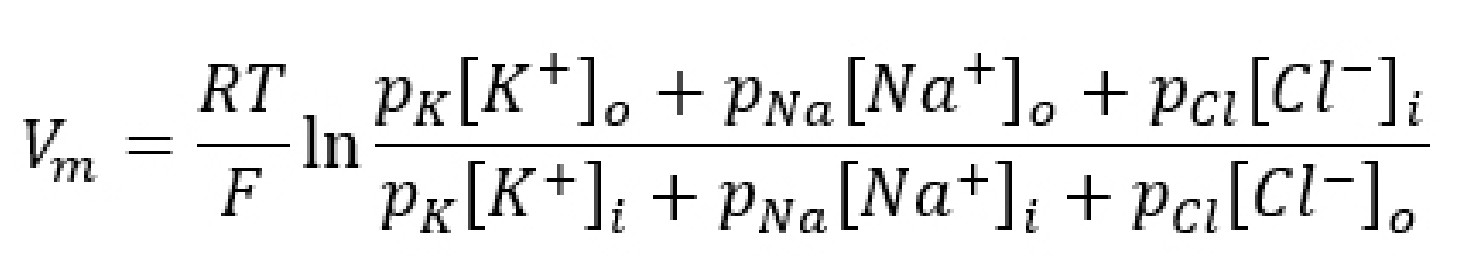
When you analyze the GHK equation, you will notice that it is essentially a combination of the Nernst equations for the equilibrium potentials for the three ions. The GHK equation also introduces a new term, the value p which stands for permeability: the ability for an ion to cross the membrane through ion channels. Permeability itself does not have a unit.
It is easier to think of permeability as the “weight” of each equilibrium potential. The higher the permeability for a given ion, the closer the Vm is to the Ex for that ion. For example, consider the value of the GHK equation for neurons at rest. Under these conditions, the permeability for K+ (pK) is 1, pCl is 0.55, and pNa is 0.04. Therefore, the resting membrane potential Vm will be closest to a combination of EK and ECl, since these two terms dominate the GHK equation. Just as a reminder, the resting membrane potential is around -70 mV, and EK is around -80 mV while ECl is around -60 mV.
However, during an action potential (described in section 4.4), permeability for sodium increases significantly. As pNa rises, the membrane potential will shift closer towards ENa, which is +55 mV. The GHK equation provides a mathematical explanation for how movement of Na+ across the membrane causes the cell to become more positive.
4.4 The action potential
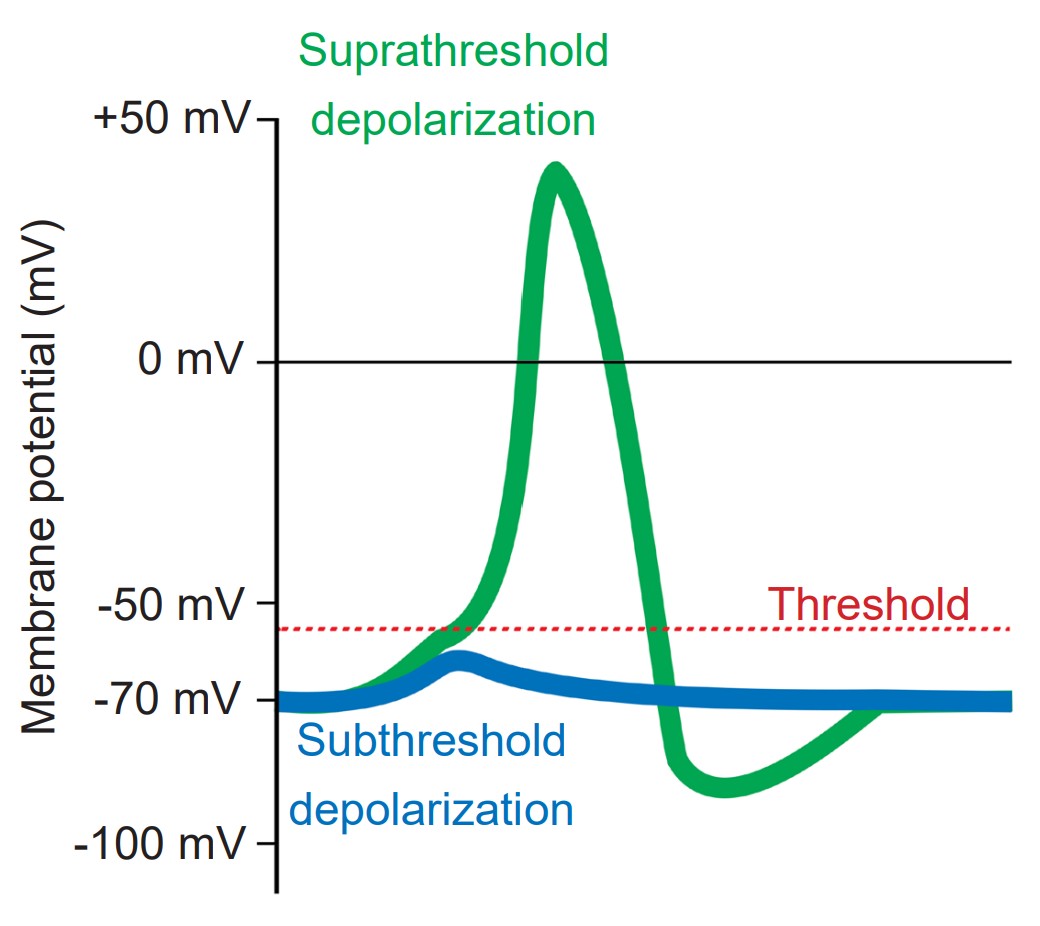
The action potential is a short-lasting (1 or 2 milliseconds), temporary change in membrane potential that can travel down the length of the axon. Action potentials are the main method of communication that neurons use, and each action potential triggers the release of neurotransmitters at the axon terminal of a chemical synapse. Action potentials are all-or-nothing responses, meaning that only a large change in potential will be able to pass the signal forward, but a small, sub-threshold change in Vm will not. Sub-threshold changes in Vm are called graded potentials.
During an action potential, the membrane potential deviates from the resting membrane potential of around -70 mV (this value can range from -90 mV to -50 mV, depending on the type of neuron) to positive potentials. When Vm goes from negative to a more positive potential, this change is described as a depolarization. Likewise, when Vm changes to a value that is more negative, it is called a hyperpolarization. The action potential has a very characteristic shape: a rapid depolarization, followed by a prolonged hyperpolarization, before repolarizing and slowly returning to the resting membrane potential over the next few milliseconds.
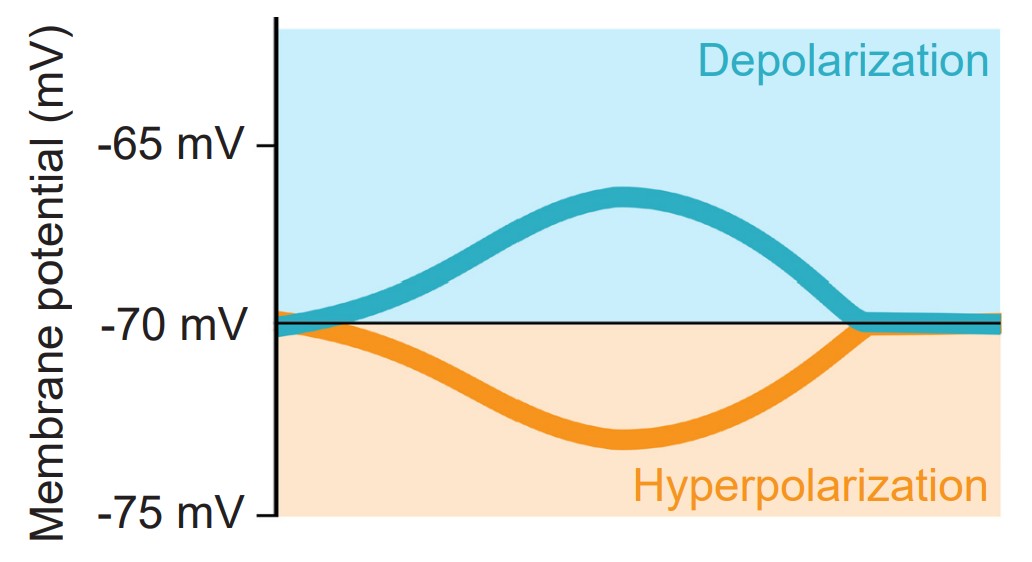
Cells regularly receive many inputs at once. To trigger an action potential, the sum of the inputs must be sufficiently depolarizing to bring the Vm to a value that is more positive than the action potential threshold. For an average neuron, the action potential threshold is around -55 mV. Anything less than the action potential threshold will fail to send the signal forward.
The change in membrane potential seen in an action potential is driven by the movement of ions, predominantly sodium and potassium, across the cell membrane through voltage-gated ion channels. An action potential takes place in 5 steps, some of which overlap in duration.
1. Depolarization from incoming neurons
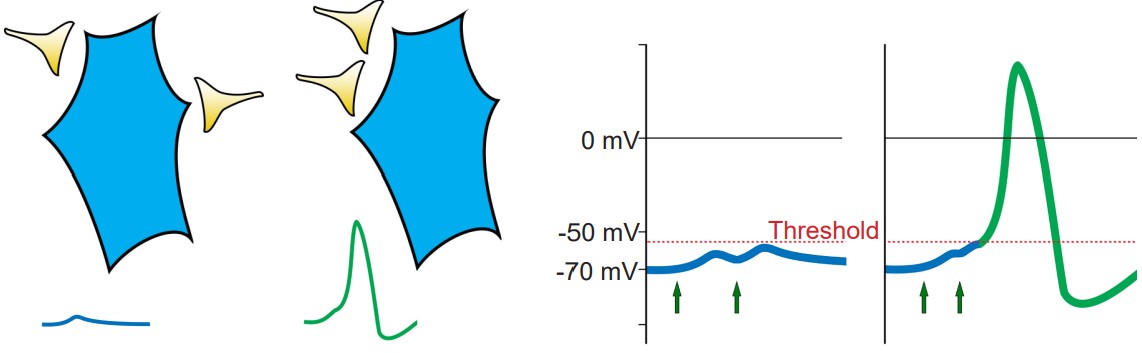
Presynaptic neurons that release neurotransmitters onto dendrites cause a small amount of ion movement via postsynaptic ligand-gated ion channels. These small deviations in membrane voltage are called postsynaptic potentials, or PSPs. Release of excitatory neurotransmitters causes small depolarizations which are called excitatory post synaptic potentials (EPSPs), while release of inhibitory neurotransmitters cause small hyperpolarizations, or inhibitory post synaptic potentials (IPSPs). These PSPs are characterized by a fast but small rising phase where the Vm changes quickly followed by a slower decay phase where the Vm resets back to resting potential.
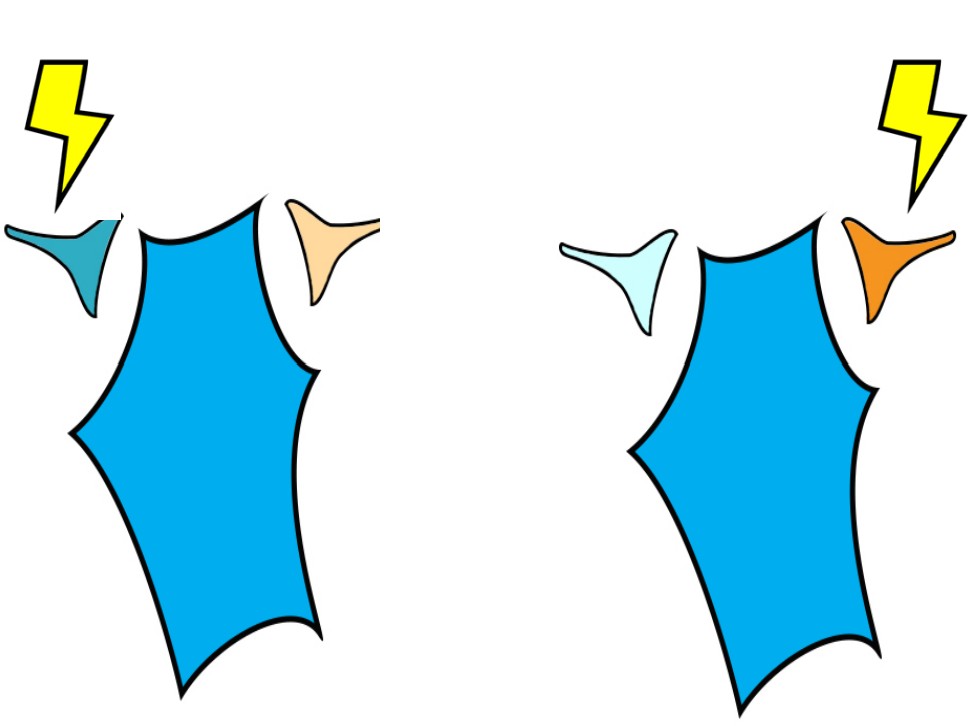
Usually, a single EPSP only causes a small amount of depolarization that isn’t sufficient for bringing Vm above the threshold potential. Reaching the threshold often requires multiple EPSPs. Multiple EPSPs are added together in one of two ways: spatial summation and temporal summation. Spatial summation happens when two small EPSPs from two adjacent inputs are triggered. Temporal summation occurs when multiple EPSPs from the same input occur close together in time. Each individual EPSP has a normal decay period, and adding a second EPSP during that decay period causes the two to add together, which may now be large enough to bring Vm above the action potential threshold. When a large enough total depolarization of membrane potential reaches the soma, an action potential is initiated at the axon hillock.
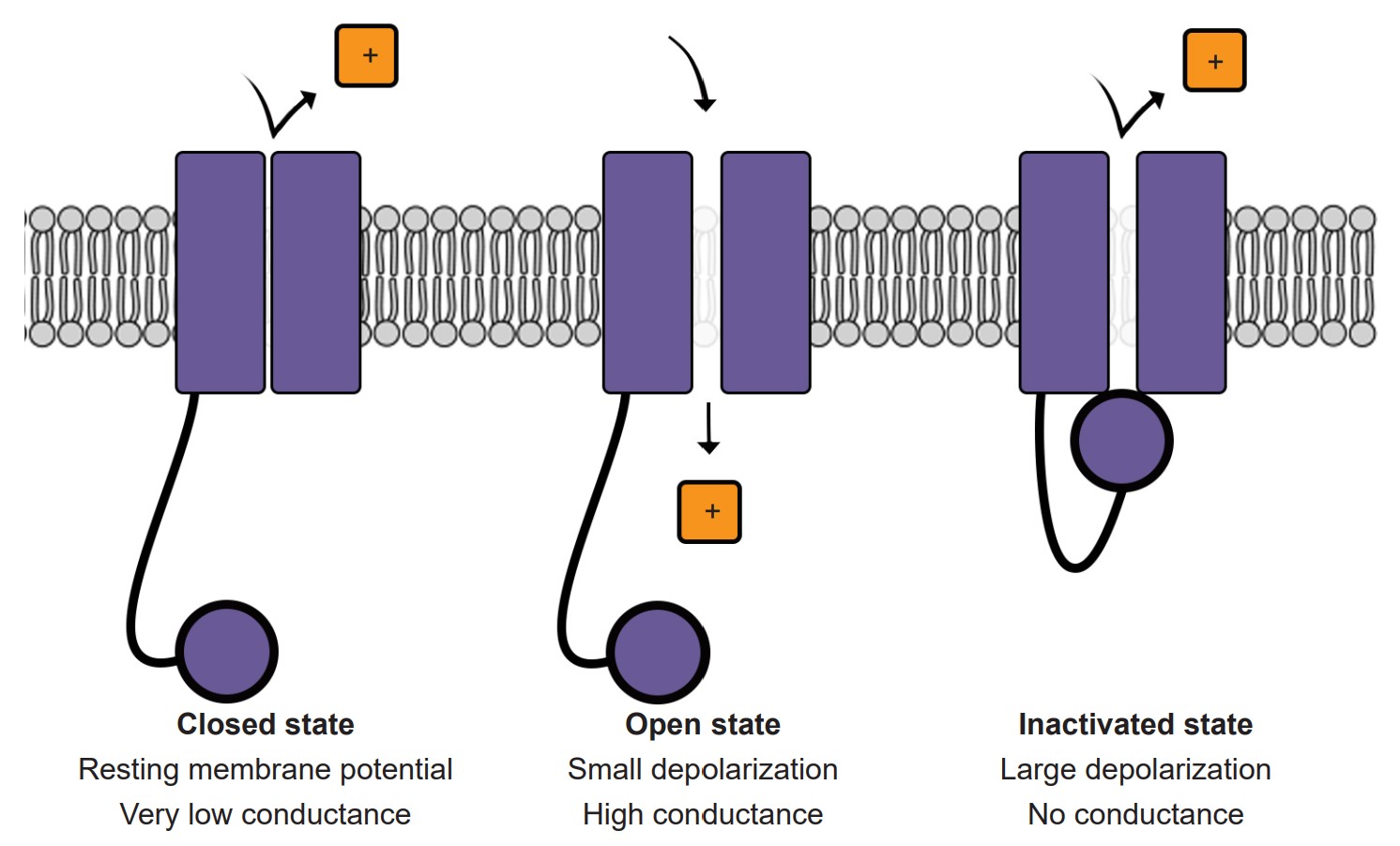
2. Opening of voltage-gated Na+ channels
At rest, the voltage-gated ion channels are almost all closed. But as the Vm depolarizes, the channels are more likely to open. At this point, we use our understanding of the electrochemical gradient to predict the movement of Na+ ions. The positively charged sodium ions are drawn to the negative potential on the inside of the cell, and the ions move from the area of high concentration (outside the cell) to an area of lower concentration (inside the cell). Both forces are in favor of Na+ entering the cell when the voltage-gated sodium channels open, and this movement of positive charges into the cell causes further depolarization. Na+ movement across the cell membrane into the cell causes the Vm to depolarize to very positive potentials. The peak of the action potential can reach +40 mV.
3. Opening of voltage-gated K+ channels
Embedded in the cell membranes are other voltage-gated channels that are selective for K+ rather than Na+. Like the voltage-gated Na+ channels, these K+ channels begin to open when the cell starts to depolarize, allowing K+ ions to move across the cell membrane.
When the cell has depolarized to about +40 mV, we can use our knowledge of the electrochemical gradient to make predictions about what happens to K+ ions. The positive potential inside the neuron repels the K+ ions (electrical gradient), and the relatively high concentration of K+ inside the cell pushes K+ out of the cell (chemical gradient). In total, both of these forces act on the K+ ions to leave the neuron. Movement of positive charge carriers out of the cell makes the interior of the cell (and Vm) more negative. K+ movement through the voltage-gated potassium channels causes the cells to become more negative than the resting membrane potential.
4. Inactivation of voltage-gated Na+ channels
Voltage-gated Na+ channels have a very complex molecular structure. In addition to having a pore through which ions can move across the cell membrane, they have an inactivation gate that can block the flow of sodium ions. When the cell membrane reaches a positive potential, the inactivation gate closes, thus preventing further movement of excitatory, depolarizing Na+ ions.
These voltage-gated Na+ channels undergo the inactivation process very rapidly, often times faster than a millisecond.
5. Deactivation of voltage-gated K+ channels
In this last step of the action potential, the main current flow is an outward current as the positively-charged K+ ions are driven out of the cell through voltage-gated K+ channels by the electrochemical gradient. When positive charges leave the cell, the interior once again becomes more negative.
Unlike the voltage-gated Na+ channels, the process of deactivation is much slower. On average, it may take these channels a few milliseconds to deactivate.
When these K+ channels deactivate, the hyperpolarizing current stops, which causes the membrane potential to gradually return to the equilibrium of resting potential.
Clinical connection: Chronic pain
Nociception is a complex sensation that the nervous system detects rapidly. Pain is the perception that the body is experiencing some kind of injury or noxious stimulus, so pain triggers a reflex that causes the person to withdraw from the painful stimulus, which decreases the severity of the damage. Pain can also create memories that discourage people from future contact with pain-inducing situations.
When we experience a noxious stimulus, that information is sent towards the central nervous system by means of action potentials. And in most cases, the detection of pain is a healthy, protective sensation.
There are, however, some people with a dysregulation in their somatosensory systems that cause them to experience pain even in the absence of injurious stimuli, a condition called allodynia. Although we still don’t understand what causes allodynia, it is known that some change in voltage-gated sodium channel properties causes an increase of excitability of pain-sensing neurons is increased.
In summary, you can also think of the shape of the action potential as consisting of three phases.
1. Depolarization. The upwards deflection (-70 mV to +40mV) of the action potential that lasts for half a millisecond. Vm becomes more positive because of Na+ ions that enter the cell through the voltage-gated sodium channels. At this step, the voltage-gated potassium channels also start to open.
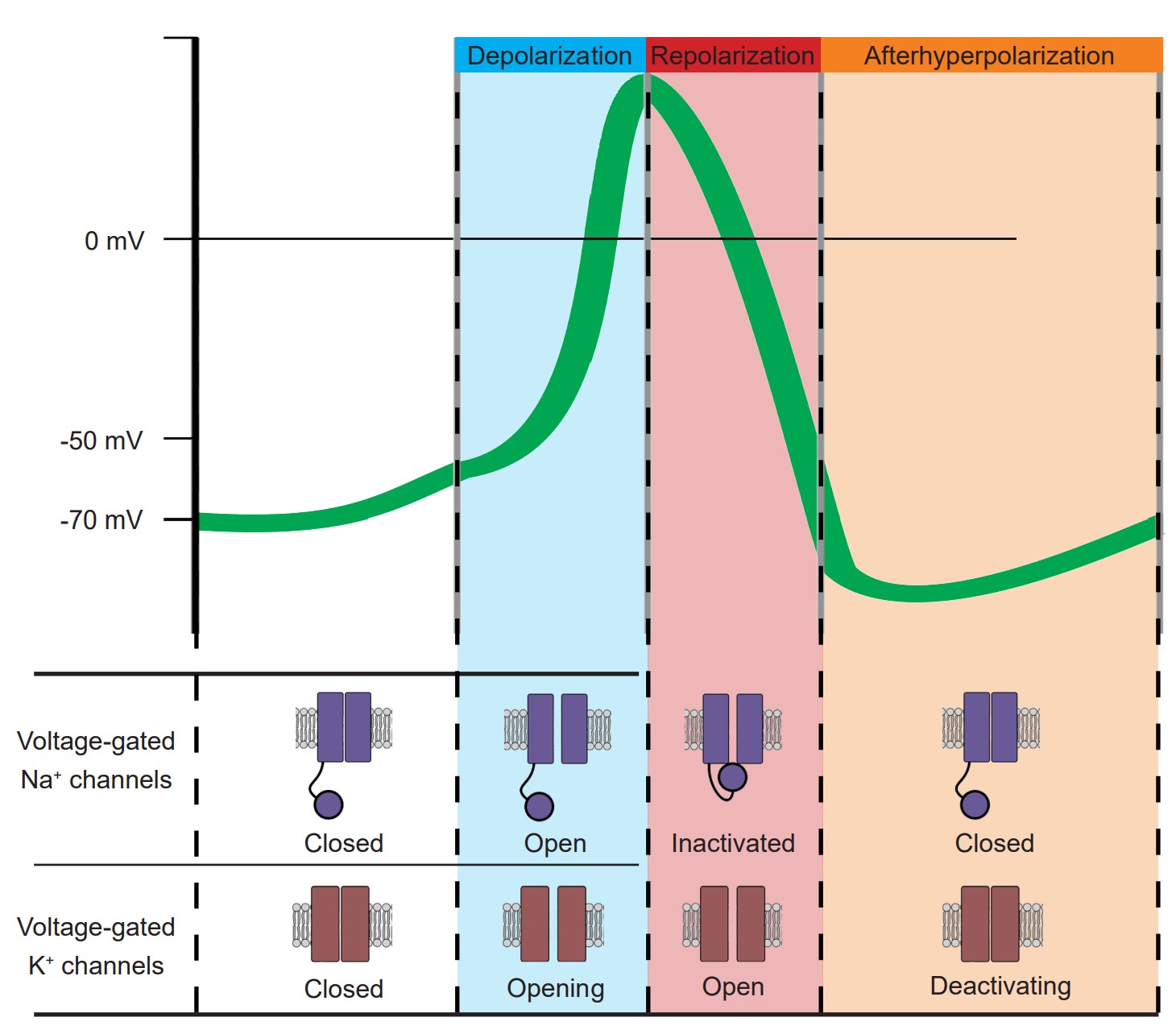
2. Repolarization. The rapid downwards deflection (+40 mV to -70 mV), also lasting for about half a millisecond. Here, the voltage-gated sodium channels have almost all inactivated, and positively-charged K+ ions are being driven out of the cell through the voltage-gated potassium channels. As positive charges leave the cell, Vm becomes more negative.
3. Afterhyperpolarization. The slow return back to the resting membrane potential (-70 mV to -80 mV and back to -70 mV ) that can last for a few milliseconds. This return back to the equilibrium of the resting state is due to the gradual deactivation of voltage-gated potassium channels.
Randomness in ion channel properties
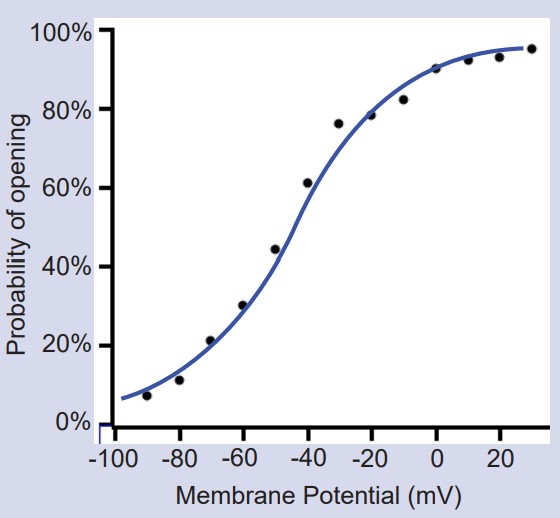
Voltage-gated ion channels do not follow a precise “if-then” flowchart in determining when to open, inactivate, or deactivate. Rather, ion channels are very probabilistic in nature. For example, consider as a voltage-gated sodium channel changes from the closed state to the open state. A single channel like this might show probabilistic behavior as follows: at -70 mV, there is a 0.1% chance to open. At -30 mV, there is now a 50% chance to open. At +20 mV, there is a 99.9% chance to transition to the open configuration.
Likewise, these channels also transition from the open state to the inactive state on a probabilistic basis as well, with the greatest probability of inactivating at depolarized potentials.
Ions moving across the membrane change the membrane potential. The best way to think about the change in Vm in response to ion movement is to consider the equilibrium potential of each individual ion that is moving. For example, ENa is +55 mV. When a voltage-gated sodium channel opens and Na+ ions are able to move across the cell membrane, the membrane potential shifts towards ENa, which is a depolarization. During the repolarization phase, when the voltage-gated K+ channels open, the potential across the cell membrane now shifts towards the equilibrium potential for K+, which is close to -80 mV.
The steps of the action potential can also be thought of in mathematical terms by examining the variables of the GHK equation. As permeability for a given ion increases, it shifts the balance of Vm more heavily towards the equilibrium potential of that specific ion. So, during the depolarization phase of the action potential when the voltage-gated sodium channels open, the permeability term for sodium (pNa) increases to as high as 20, which causes Vm to move towards ENa (+55 mV). And during the repolarization phase, voltage-gated potassium channels open, while the voltage-gated sodium channels are inactived. When this happens, pK increases, pNa drops to zero, and as a result, the Vm shifts towards the equilibrium potential for potassium (-80 mV).
In the moments following the beginning of an action potential, there is a time window where a second action potential cannot be fired. This time window, about half a millisecond in duration, is called the absolute refractory period. On a molecular level, the absolute refractory period exists because the voltage-gated sodium channels are inactivated, which prevents them from being able to pass any more inward excitatory current. The absolute refractory period may last for up to a millisecond.
A few milliseconds after the end of the absolute refractory period begins a phase called the relative refractory period. During this phase, it is more difficult to fire an action potential compared to the resting condition. At the level of the receptors, the relative refractory period exists for two main reasons. First, only some of the voltage-gated sodium channels have “reset” from their inactive state. Second, many of the voltage-gated potassium channels are still in the open state, which allows the movement of K+ ions, causing the neuron to rest at a more negative potential. Also, increased movement of K+ ions hinders depolarization, as the GHK equation demonstrates, since potassium movement pulls membrane potential closer towards the equilibrium potential for K+. This relative refractory period lasts as long as the afterhyperpolarization, and therefore exists on a gradient: the sooner after the repolarization phase of the first action potential, the more difficult it will be to reach threshold for a second action potential.
4.5 Movement of the action potential
Up to this point, we have only considered a single piece of cell membrane and how it responds during an action potential. For an action potential to travel from the axon hillock to the axon terminal, this temporary change in Vm must physically move down the length of the axon. The ability for the action potential to travel is made possible because the Na+ ions that enter through voltage-gated sodium channels are not restricted to the cytoplasmic volume beneath the ion channels. These Na+ ions behave just like any other substances that are in an area of high concentration: they move towards areas of low concentration. As Na+ rushes into the cell, they follow the chemical gradient and quickly move toward areas of low concentration, which is the next section of membrane. When they do so, the next patch of the membrane becomes depolarized. This depolarization causes the next set of channels to open, which results in even more sodium influx. From here, the process repeats down the axon. The action potential is a chain reaction that starts at the axon hillock and doesn’t stop until the axon terminal.
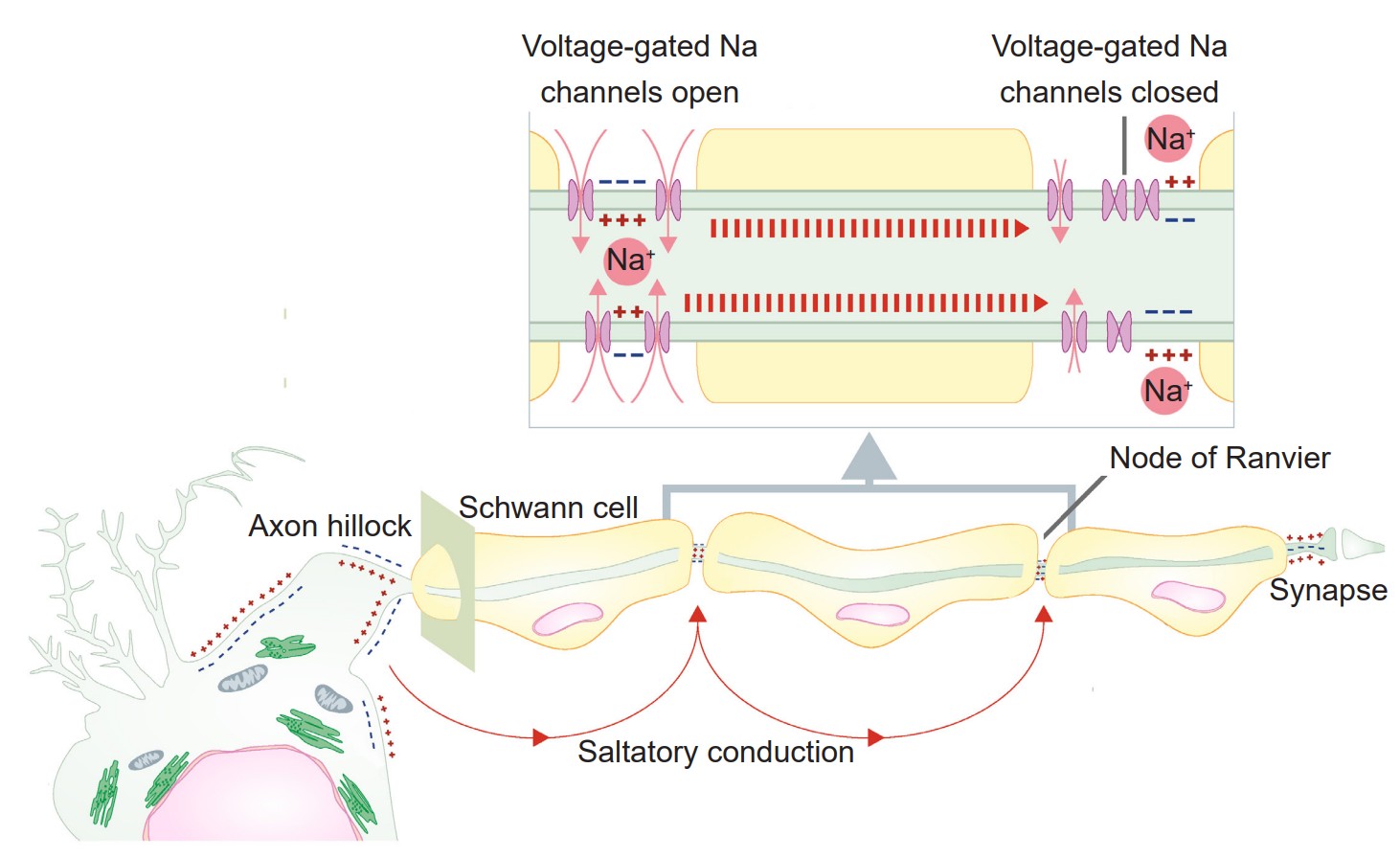
An action potential is like a wave. It only moves in one direction, and there are two main reasons why:
1. First, sodium ions move down their concentration gradient. The Na+ ions that enter through a voltage-gated sodium channel are less likely to want to move in the reverse direction, since this area currently has a relatively high Na+ concentration. Instead, they will move towards the area with a low concentration of ions, which is the forward direction.
2. Secondly, the previous patch of membrane is in the absolute refractory period, which makes it impossible for an action potential to travel backwards. During this time, many voltage-gated sodium channels are still inactivated, and most voltage-gated potassium channels are open.
Analgesia and motor signaling
A trip to the dental office sometimes requires an injection of an analgesic like lidocaine before the dentist performs any otherwise-painful procedures. On a molecular scale, lidocaine is an inhibitor of voltage-gated sodium channels. By blocking these channels, lidocaine prevents action potentials from traveling up the afferent pathway, preventing incoming sensory inputs from the teeth and gums from reaching the central nervous system. When these signals are blocked, we don’t feel any drilling.

Just as incoming signals can be inhibited through voltage-gated sodium channel inhibitors, so can outbound signals – sometimes with deadly consequences. Through evolution, nature came across a variety of voltage-gated sodium channel inhibitors that function as poisons to prevent predation. Because the efferent pathway is used to signal components of the motor system, blocking downward signals can lead to paralysis. One family of fishes, the pufferfish, may have a very high concentration of a deadly voltage-gated sodium channel inhibitor called tetrodotoxin, or TTX. This toxin is produced by a bacteria that live symbiotically in pufferfish organs. Ingestion of TTX prevents neurons from communicating. One efferent signaling pathway, the phrenic nerve, signals the diaphragm to move up and down. Without proper medical treatment, people exposed to doses as small as 1 milligram of TTX can die of respiratory failure in hours.
Despite these tremendous risks, pufferfish is a delicacy in Japan. The dish, called fugu, can cause numbness of the lips and even a mild intoxication.
The role of myelin
Previously, in discussing the cellular anatomy of the neurons, we described myelin, layers of lipid that are sometimes thickly wrapped around the axon of the neuron. Myelin increases conduction velocity, the speed at which an action potential travels down the length of the axon. Myelin works by physically blocking leak potassium channels in the cell membrane. By covering these channels, positive charges become unable to exit the cell, which causes the signal to move more rapidly.
A myelinated axon still requires some influx of sodium ions for the signal to travel, however. This influx occurs at the nodes of Ranvier, the unmyelinated segments of the axon. These areas are very dense with voltage-gated sodium channels. Because the changes in electrical charge are detected at intervals, we sometimes say that the signal is passed by saltatory conduction (saltare is the Italian word for “jump”).
In this chapter, we described the molecular and cellular underpinnings of the action potential, the main method of signaling that the nervous system uses for communication. In the following chapter, we will explain how neurons talk to one another using those electrical signals in combination with chemical signals called neurotransmitters.
Image Credits
4.4. OpenStax / CC BY (https://creativecommons.org/licenses/by/4.0) https://commons.wikimedia.org/ wiki/File:Openstax_college-physics_22.5_magnet-pole-attraction.jpg
4.5 (diffusion fig BruceBlaus [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)]
4.18 Correa AM, Bezanilla F. Gating of the squid sodium channel at positive potentials: II. Single channels reveal two open states. Biophys J. 1994 Jun;66(6):1864-78. doi: 10.1016/S0006-3495(94)80980-4. PMID: 8075324; PMCID: PMC1275912.
4.19 https://commons.wikimedia.org/wiki/File:Propagation_of_action_potential_along_myelinated_ nerve_fiber_en.svg modified by Austin Lim
4.20 https://upload.wikimedia.org/wikipedia/commons/4/4e/Pufferfish_%28Butete%29.jpg
Chapter 5: Signaling Between Neurons
Previously, we described the electrical properties of a single neuron. A lone neuron can send action potentials as a means of communication, but cells become much more interesting when they have partners to talk to. The nervous system of the worm C. elegans is only 300 neurons, and yet it is complex enough to engage in moderately intricate behaviors like responding to repellant or attractant odors, social feeding, and long-term learning. The human brain, with its 86 billion neurons, can engage in these behaviors and so many more – only because of communication between the different neurons in the brain.
A lone neuron can send action potentials as a means of communication, but cells become much more interesting when they have partners to talk to. The nervous system of the worm C. elegans is only 300 neurons, and yet it is complex enough to engage in moderately intricate behaviors like responding to repellant or attractant odors, social feeding, and long-term learning. The human brain, with its 86 billion neurons, can engage in these behaviors and so many more – only because of communication between the different neurons in the brain.
In this chapter, we will focus on the molecular-level features of communication between neurons, starting from the anatomical differences between synapses.
5.1 Electrical vs. chemical synapses
The synapse is not a part of the structure of a neuron, but rather the site of close proximity between two communicating neurons. There are two main types of synapses: electrical and chemical.
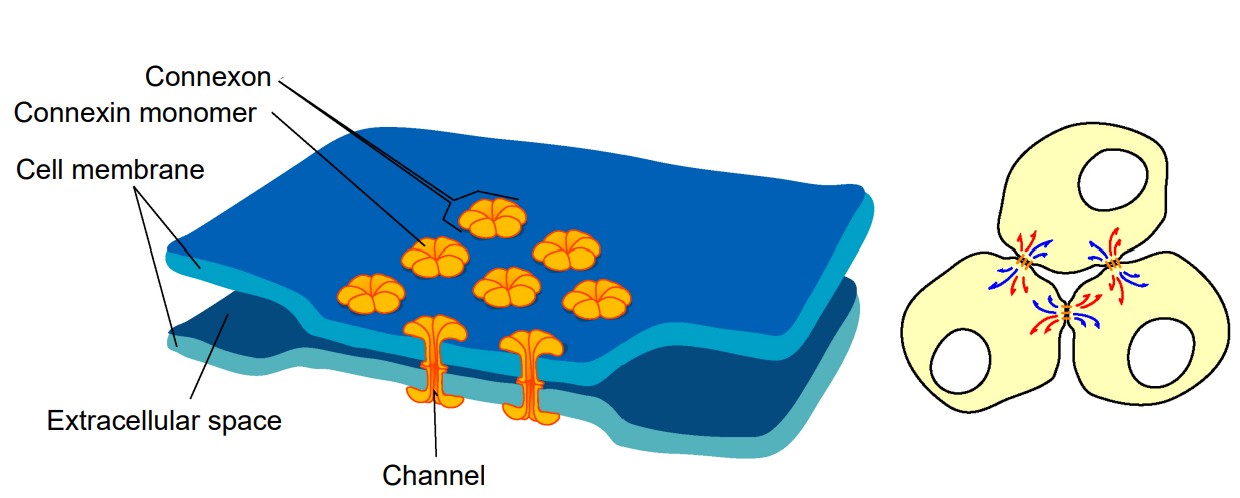
Electrical synapses
Electrical synapses are the simpler of the two types of synapses. In an electrical synapse, the main driver of communication between two neurons is a change in potential, and the carrier of charge is almost always an ion. The electrical synapse simply means that the cells share their cytoplasm with an adjacent cell. Electrical synapses are what Camillo Golgi imagined when he proposed the reticular theory of nervous system organization. Oftentimes an entire network of many hundreds of neurons is connected by these synapses.
Imagine two neurons that are connected by an electrical synapse. First of all, both of them are complete cells on their own. Each one contains a complete plasma membrane surrounding the neuron, a nucleus, and all the individual organelles needed to carry out that cell’s basic life processes. Electrical synapses share the cytoplasm between the two connected cells, so ions, ATP, and larger signaling molecules and proteins are able to move between the two cells. For this to happen, there exists a specialized physical channel between the two that allows for the passage of cytoplasm called a connexon or hemichannel. Each hemichannel itself is made up of six transmembrane proteins called connexins (you can remember the difference between the channel and the individual protein because proteins often end with the letters -in). When two connexons contact each other, they interact closely with each other and form the gap junction, which is the structure that connects neurons electrically.
Neurons that are connected by electrical synapses are remarkably close to each other. The synaptic gap between the two electrically connected neurons is about 3.5 nanometers. Logically, the neurons must be close since the hemichannels are like a physical “bridge” between the two cells.

Electrical synapses are capable of passing information bidirectionally. This means that a signal does not always move sequentially from the presynaptic cell to the postsynaptic cell. Rather, ions and signaling molecules are free to move through the connexons in either direction. Also, each cell within an electrically-coupled network can receive inputs at any of the cells, making it able to detect several signals at once – the same way a huge satellite dish can detect more signals than a small dish.
Electrical synapses likely evolved because of evolutionary pressures that selected for speed. These synapses can pass signals as fast as electrical charges can move through an electrolyte-rich fluid like cytoplasm, which is almost instantaneous. Therefore, an escape reflex that is made up of communication across electrical synapses is advantageous for animals that need to escape predators. For example, crayfish exhibit a reflexive abdominal flexion response when exposed to threatening stimuli, causing the animal’s body to dart away from a threat within a fraction of a millisecond. Comparing across the phylogenetic tree, electrical synapses are often found in less complex organisms, including arthropods such as insects and crustaceans, where such reflexes are likely more critical for survival.
Another advantage of electrical synapses is that they can form a large network of interconnected neurons with synchronized activity. For example, neuroendocrine cells in the hypothalamus are connected by electrical synapses. When the “go” signal arrives, all the cells depolarize at once, which can result in the massive release of hormones into the bloodstream. A network can also cause sudden, powerful inhibition. Like an angry mob of people chanting, a network of electrical synapses connecting inhibitory interneurons allows the network to send an immediate “shut-down” signal under specific circumstances.
Clinical connection
Charcot-Marie-Tooth (CMT) disease Charcot-Marie-Tooth (CMT) disease is a rare genetic disorder that damages parts of the peripheral nervous system including the motor nerves, resulting in muscle weakness and difficulty with walking, and the sensory nerves, causing some to experience abnormal sensations such as tingling or pain in their extremities. These symptoms are characteristic of signal transduction failure resulting from deficits in myelin. One type of connexin protein, called Cx32, is heavily expressed in Schwann cells, the glia that produce myelin in the PNS. Mutations in the gene that codes for Cx32 are associated with the X-linked form of CMT disease, and knocking-out the gene in experimental mice cause the mice to express similar symptoms as human CMT.
Chemical synapses
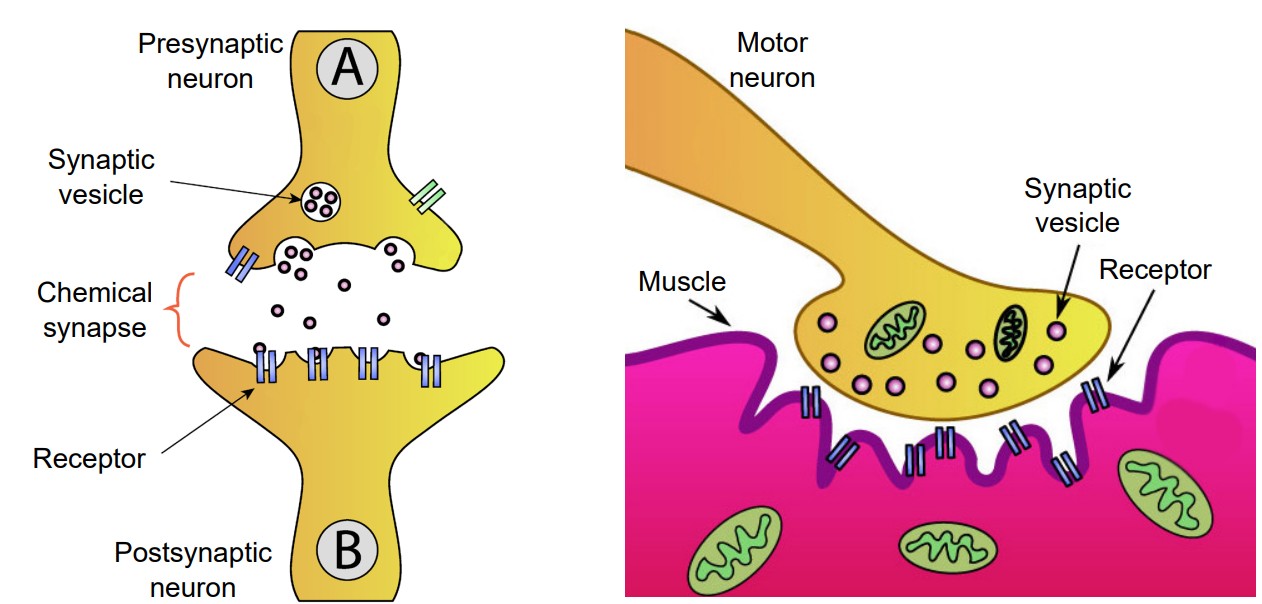
At a chemical synapse, a signaling molecule is released by the presynaptic cell to influence the postsynaptic cell. These signaling molecules, generally called neurotransmitters, are synthesized or stored by neurons. After being released, these neurotransmitters diffuse randomly across the synapse, where they are able to affect nearby neurons once the chemical binds to its corresponding receptor.
Since chemical synapses do not rely on a direct physical protein “tunnel” to connect the two neurons, the distance between the two cells can be much larger. On average, a chemical synapse is a distance of about 20-40 nanometers, roughly a thousand times smaller than the diameter of a human hair.
A chemical synapse can pass a variety of signals, depending on the neurotransmitter and the receptor. For example, some signals are directly excitatory and allow positively charged cations to enter the neuron causing depolarization. Other signals are hyperpolarizing, and therefore inhibitory. And yet other signals are much more complex, inducing changes in protein expression that can modify cellular excitability over the course of minutes or hours.
Because of the complexity of the signals that chemical synapses can convey, evolutionary development through time has allowed for a tremendous variety of responses. Chemical synapses allow for fine-tuning of neural networks, giving these nervous systems a larger range of possibilities. The nervous systems of “higher” organisms like humans tend to have several chemical synapses since these signals are likely necessary for complex behaviors and cognition.
Many chemical synapses exist between the axon of one neuron and the dendrite of another neuron. One specific type of chemical synapse refers to the space between a motor neuron and muscle tissue, and this is called the neuromuscular junction, or NMJ. When the chemical signaling molecule acetylcholine (ACh) is released by the presynaptic motor neuron, it is detected by receptors that are expressed on the muscle. The release of ACh causes contraction of the muscle.
5.2 Properties of vesicles
Types of vesicles
Molecules of neurotransmitters are often stored in synaptic vesicles before being released. Synaptic vesicles are tiny spheres of lipids just like the cell membrane. These vesicles can be roughly characterized into one of two classes:
1. Small vesicles. These vesicles have a diameter of 40 nanometers and a volume of about 30 cubic microns. Given the size of neurotransmitters, we can estimate at somewhere on the order of thousands to tens of thousands of molecules of neurotransmitter can be stored in each vesicle. Small vesicles store most of the neurotransmitters we most often think of, including glutamate, GABA, dopamine, and norepinephrine, for example. Small vesicles are almost always exclusive found in the axon terminals.
2. Large dense-core vesicles. These vesicles are much larger than small vesicles, with a range of diameter from 100 to 250 nanometers. They store peptides such as dynorphin or enkephalin, which have chemical structures much larger than the other neurotransmitters. Since these peptides are packaged into their vesicles near the nucleus, large dense-core vesicles can be found in the cell bodies and all along the axons in addition to the axon terminal.
Loading of vesicles
Vesicles need to be filled with molecules of neurotransmitter before release into the synapse. In small vesicles, filling is only made possible through the action of giant transmembrane proteins called vesicular transporters. These are protein complexes that span the vesicular membranes, with one side facing the intracellular space and other facing the inside of the vesicle. Their main function is to take molecules of neurotransmitter from the intracellular space of the axon terminal and pump them into vesicles.
Electron microscopy
Electron microscopy is a technique that allows for the imaging of subcellular structures that are on the order of nanometers, such as synapses, small vesicles, and large dense core vesicles.
Many of the vesicular transporters are named based on the neurotransmitters that they are capable of recognizing and transporting. Some have a single substrate, such as vesicular GABA transporters (VGAT) which move GABA, vesicular glutamate transporters (VGluT) which move glutamate, and vesicular acetylcholine transporter (VAChT) which moves acetylcholine into vesicles. Others recognize a broad class of neurotransmitters, such as the vesicular monoamine transporters (VMATs), which are responsible for moving monoamines such as dopamine and serotonin into the vesicles.
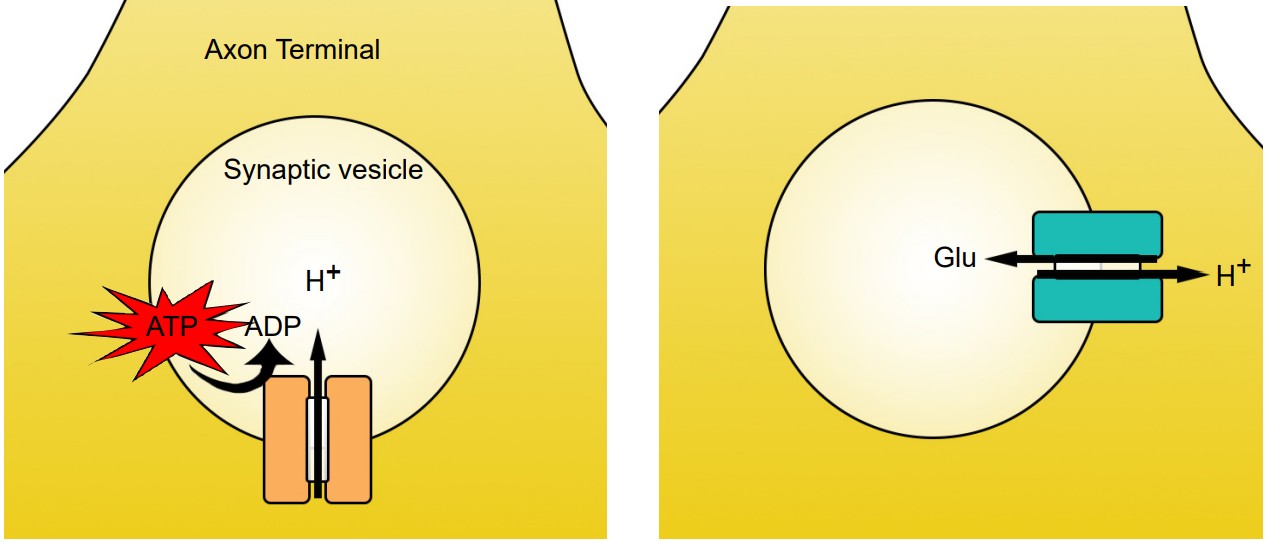
Vesicular transporters are able to function because the interior of the vesicle is highly acidic compared to the interior of the cell. Vesicles have a high concentration of H+ ions (protons) because of the action of the transmembrane enzyme vesicular ATP-ase, or v-ATP-ase. These membrane-embedded proteins utilize the molecular energy contained in ATP to concentrate H+ ions in the intravesicular space. For each molecule of ATP used, one proton gets pumped into the vesicle.
Vesicular transporters pump molecules of neurotransmitter against their concentration gradients, which is an energetically difficult task. To have enough energy, the vesicular transporters use the high intravesicular concentration of H+ to move molecules of neurotransmitter across the vesicular membrane. When a proton moves from an area of high concentration to low concentration, energy is generated. The vesicular transporters use this energy to push neurotransmitter in. Because H+ ions move opposite of the neurotransmitter molecules, vesicular transporters are called antiporters. Transporters have slightly different stoichiometries, as it requires two protons to move a single molecule of dopamine, while the energy from a single proton is sufficient to transport GABA or glutamate.
Location of vesicles
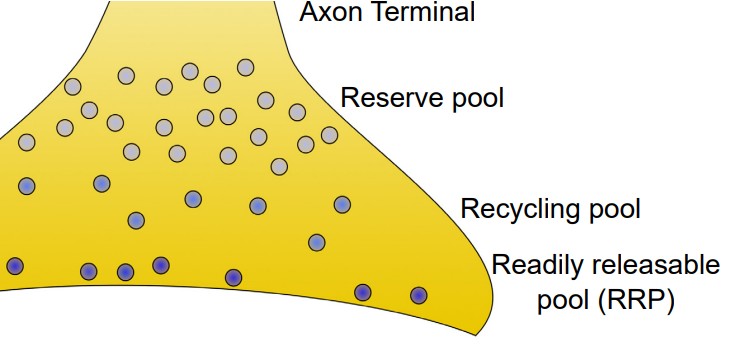
Synaptic vesicles can be found in one of three places at the axon terminal.
1. Readily releasable pool (RRP). These vesicles are located close to the cell membrane at the axon terminal. In fact, many of them are already “docked,” meaning that their coat proteins are already interacting closely with the proteins on the inside of the cell membrane. When the depolarizing charge of an action potential reaches the terminal, these vesicles at the RRP are the first ones that fuse with the cell membrane and release their contents.
2. Recycling pool. These vesicles are the ones that have been depleted due to release. They are currently in the process of being refilled or reloaded with neurotransmitter. They are farther from the cell membrane, and the protein machinery is not primed for release, so it requires a more intense stimulation to release the contents of these vesicles.
3. Reserve pool. These vesicles are the farthest from the surface of the cell membrane, and most vesicles are held in this reserve pool. For these neurons to be released, very intense stimulation is required. Reserve pool vesicles may not even be recruited for release under physiological conditions.
Release of vesicles
At a chemical synapse, the process of neurotransmitter release is very tightly regulated. If there were no mechanisms to control the release of chemicals at the synapse, nerve cells would deplete their entire stock of neurotransmitter. The signals that trigger muscle contraction at the NMJ would cause constant muscle tension. All sorts of brain signals would be active, and over-excitation would cause toxicity. Needless to say, regulated control of neurotransmitter release is a normal and essential part of nervous system function.
Regulation of release depends on several proteins that are important parts of the process. These proteins are often embedded within cell membranes of the vesicles or the neuronal membrane.
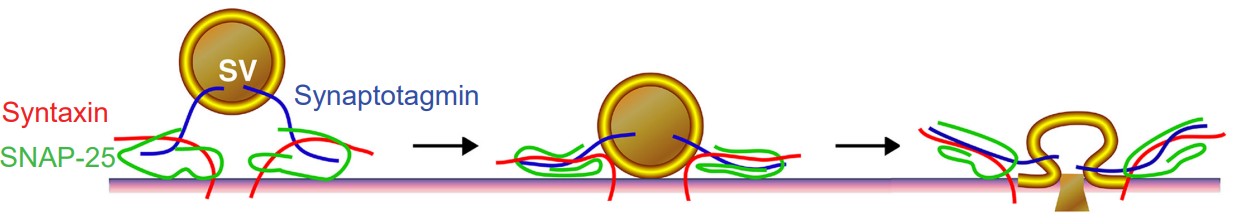
1. V-SNAREs are the proteins expressed on vesicles (v for vesicle). Synaptobrevin and synaptotagmin are two specific v-SNARE proteins that are involved during synaptic release.
2. T-SNAREs are proteins expressed on the cytoplasmic side of the axon terminal. The inside of the cytoplasm is the “target” for the vesicle (The t in t-SNARE). Syntaxin and synaptosomal nerve-associated protein 25, or SNAP-25 for short, are t-SNAREs that function during vesicular fusion.
Clinical connection: Botulism
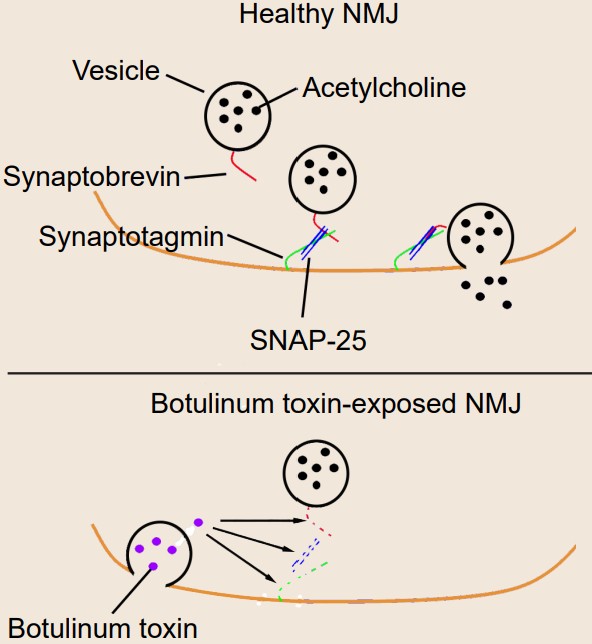
Botulism is a deadly condition that results from exposure to the spores produced by the bacteria Clostridium botulinum. Toxic spores can be found in the soil, contaminated foods, or water. The toxin itself is one of the most potent agents known to man – exposure to concentrations as low as 2ng/kg is lethal. The most common symptoms include muscle weakness or paralysis, especially the muscles of the face or the limbs. For about 5% of people who develop botulism, death results from paralysis due to respiratory failure.
Botulinum toxin is known to selectively cleave the proteins that comprise the SNARE complex. There are a few specific types of botulinum toxin with slightly different intracellular targets, but the result is the same on the molecular level: prevention of vesicular fusion eliminating neurotransmitter signals.
Despite being one of the deadliest toxins so far identified, millions of people pay to have a preparation of toxin called “Botox” injected into their face. For most, the injection of botox is a cosmetic procedure that can reduce the appearance of wrinkles by paralyzing the muscles. Botulinum toxin is also used medically for conditions resulting from excessive neurotransmitter release, such as muscle spasms, excessive sweating, or migraine.
Fusing of vesicles
The last step of neurotransmitter release is the fusing of the cell membrane. In order to release their chemical contents into the synapse, vesicles need to fuse with the cell membrane. As the vesicular membrane merges with the interior of the neuronal membrane, the contents of the vesicle become exposed to the extracellular space. Only then are the neurotransmitters capable of activating receptors.
One of the key proteins required for vesicular fusion is the vesicle-embedded V-SNARE synaptotagmin. This protein is capable of detecting elevated levels of Ca2+ in the axon terminal. As it turns out, an elevation of Ca2+ in the intracellular space is the “go ahead” signal that causes neurotransmitter release.
The concentration of intracellular calcium, generally in the range of 100 nM, is much lower than the concentration outside the cell. Embedded in the cell membrane of the axon terminals are transmembrane proteins called “voltage-gated calcium channels” or VGCCs. As their name suggests, they function very similarly to the voltage-gated sodium channels described in previous chapters: they are large protein complexes that normally remain closed, but when the surrounding neuronal membrane becomes depolarized, they physically change conformation and open up, allowing ions to move across the cell membrane. These VGCCs selectively pass only Ca2+ ions. The electrochemical gradient causes these Ca2+ ions to enter into the cell.
As the change in membrane potential travels down the length of the axon (the action potential), it causes a depolarization at the terminal, triggering calcium entry via the VGCCs. Ca2+ at the terminal binds with synaptotagmin. The v-SNAREs and the t-SNAREs interact with one another in the presence of Ca2+, forming a molecular structure called a SNARE complex. The SNARE complex looks a lot like two twist ties that are wound tightly together. As they twist tighter together, it causes the vesicle membrane to approach the inside of the cell membrane, which results in vesicular fusion.
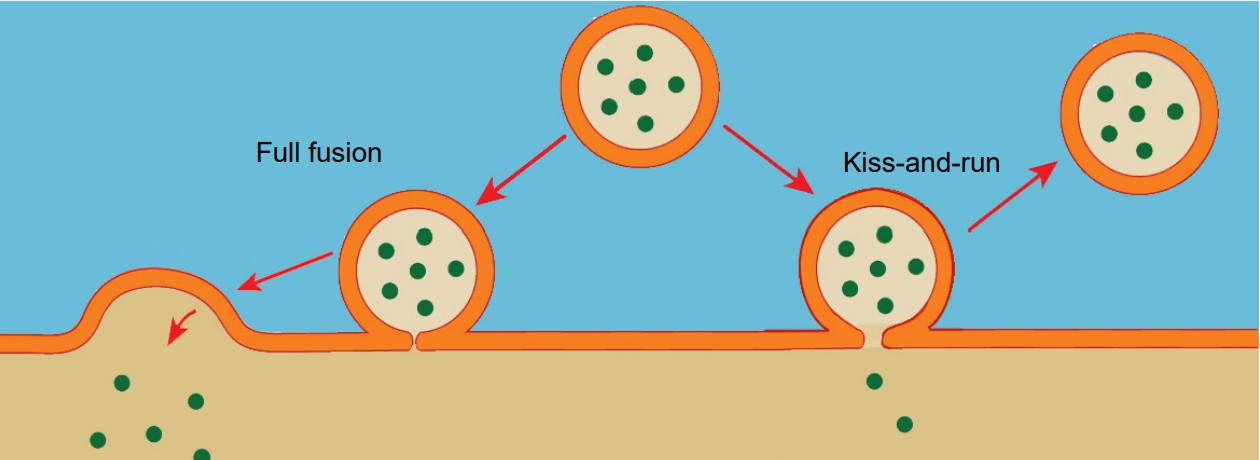
Vesicles are capable of fusing in at least two different ways.
1. Full fusion. A vesicle that undergoes full fusion experiences total exocytosis. The vesicular membrane becomes completely integrated with the cellular membrane, and all of the neurotransmitter spills into the synapse.
2. Kiss-and-run. This method of neurotransmitter release is incomplete fusion. The vesicle only partly connects with the interior surface of the cell membrane, and only a limited number of neurotransmitter molecules are able to enter the synapse via diffusion.
5.3 Receptors
Receptors are proteins that are capable of sending a signal to change the function or activity of a neuron. Most receptors that function in neurotransmission are large transmembrane proteins. On the extracellular surface is a specific series of amino acid residues called the active site. The active site, also called the orthosteric site, is shaped to allow molecules of neurotransmitter to bind to the receptor.
Receptors are classified into one of two main categories.
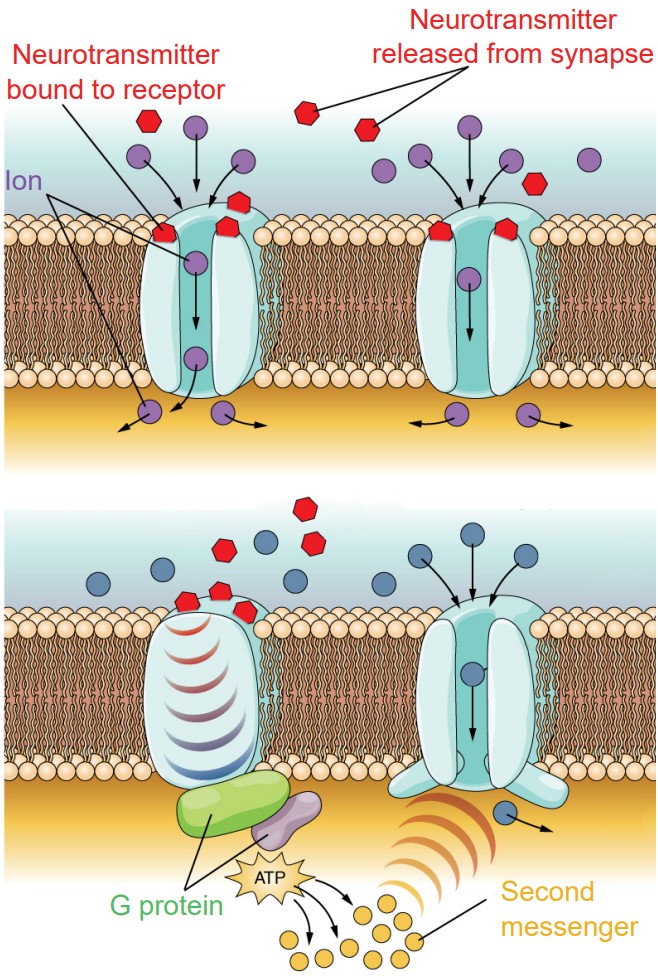
1. Ionotropic receptors. Physically, ionotropic receptors are transmembrane proteins with a large-diameter pore through which ions can pass. These channels only open when a molecule of chemical binds to the active site on the extracellular side of the protein. These chemicals are also called ligands, and so ionotropic receptors are also called ligand-gated ion channels. Once a neurotransmitter activates the ionotropic receptor, ions will move based on the electrochemical gradient for that ion. As a result of ion movement, the cell’s membrane potential will change. For example, nicotinic acetylcholine receptors are ligand-gated sodium channels, so when these receptors are activated by a ligand like acetylcholine, sodium ions enter into the cell causing depolarization and excitation.
Ionotropic receptors are able to induce a change in membrane potential very rapidly, on the scale of milliseconds.
Due to the nature of the amino acid residues that make up the pore of ionotropic receptors, they can be very selective for certain ions. For example, negatively charged residues lining the inside of the pore repel negatively charged Cl- ions while allowing positively charged cations to pass through the channel.
2. Metabotropic receptors. These receptor complexes cause the cell to change its metabolism in a way that leads to either excitation or inhibition. Ions do not pass through these receptors. Instead, metabotropic receptors use the actions of G proteins, proteins which induce changes in neuronal excitability through the action of second messenger signaling molecules.
Physically, metabotropic receptors are transmembrane proteins that contain 7 alpha-helix motifs that pass through the cell membrane. The N-terminus of the protein is extracellular, and the protein “weaves” back and forth across the cell membrane, resulting in a protein with three extracellular loops and three intracellular loops. Because of this shape, these receptors are also called seven-transmembrane receptors, or 7-TM receptors.
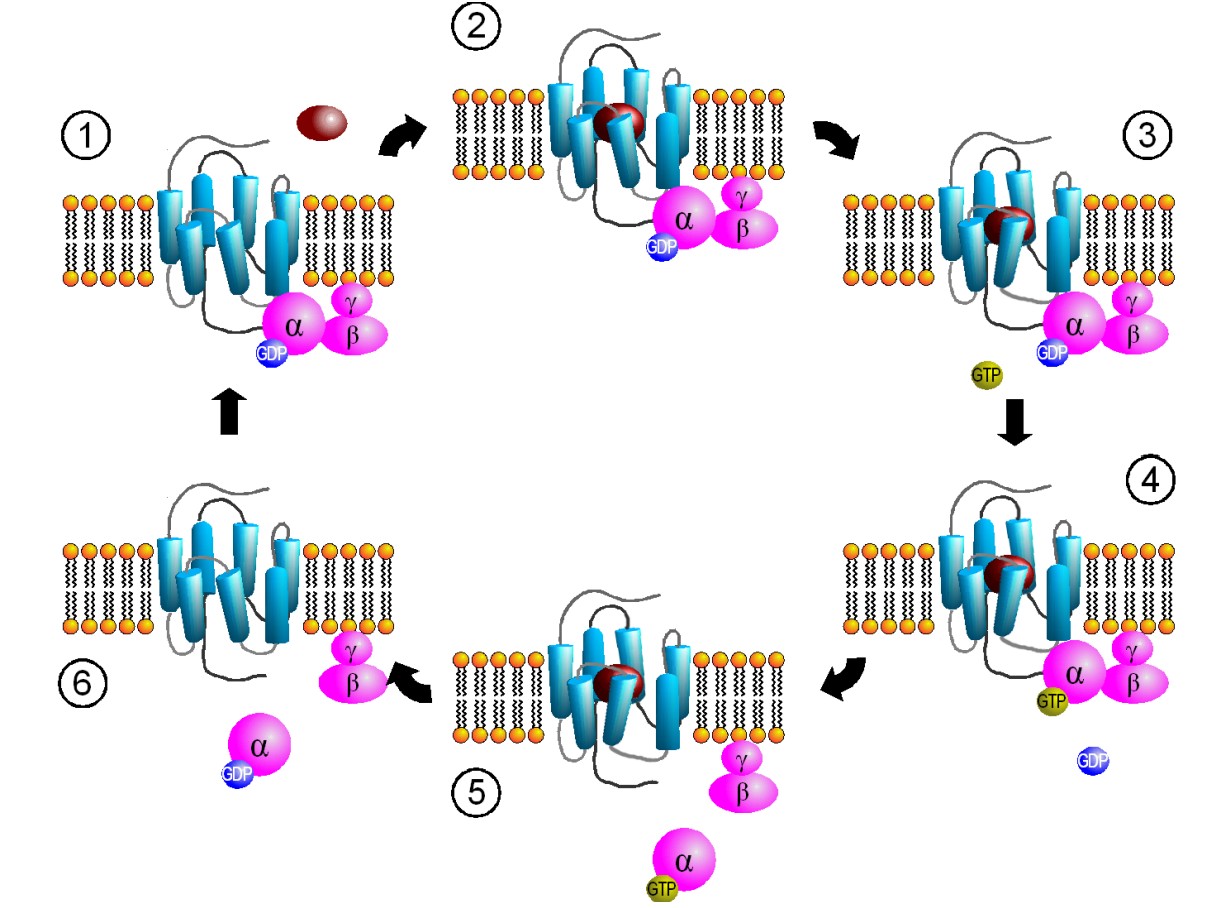
Another name for these receptors is “G protein-coupled receptors”, or GPCRs. Metabotropic receptors are physically linked to proteins called G proteins, which exist on the inner surface of the cell membrane. Functionally speaking, these G proteins are capable of binding to molecules of guanosine triphosphate (GTP) or guanosine diphosphate (GDP). Chemically similar to ATP, GTP can function as a source of energy. G proteins themselves exhibit catalytic activity of GTP. This means that they are capable of breaking down GTP into the less-energetic GDP. When GTP is bound to the GPCR, the receptor is active. When this molecule is hydrolyzed into GDP, the receptor becomes inactive.
Some G proteins are heterotrimeric, meaning that they are made up of three different subunits, alpha, beta, and gamma. The GTP binding sites and catalytic sites are found on the alpha subunit, the largest of the three subunits. Usually, the alpha subunit becomes soluble after activation, while the beta / gamma complex stays embedded in the neuronal membrane. The G alpha subunit exists in different varieties.
Gαs. When a neurotransmitter activates a GPCR coupled with the Gαsprotein, the Gαs protein is excitatory (The “s” stands for stimulatory).
Binding of a ligand to the active site of Galphas-coupled GPCRs results in increased activity of the enzyme adenylate cyclase (AC). AC itself is an enzyme that creates a second messenger, a molecule called cyclic AMP (cAMP). Elevated levels of cAMP activate an enzyme called protein kinase A (PKA).
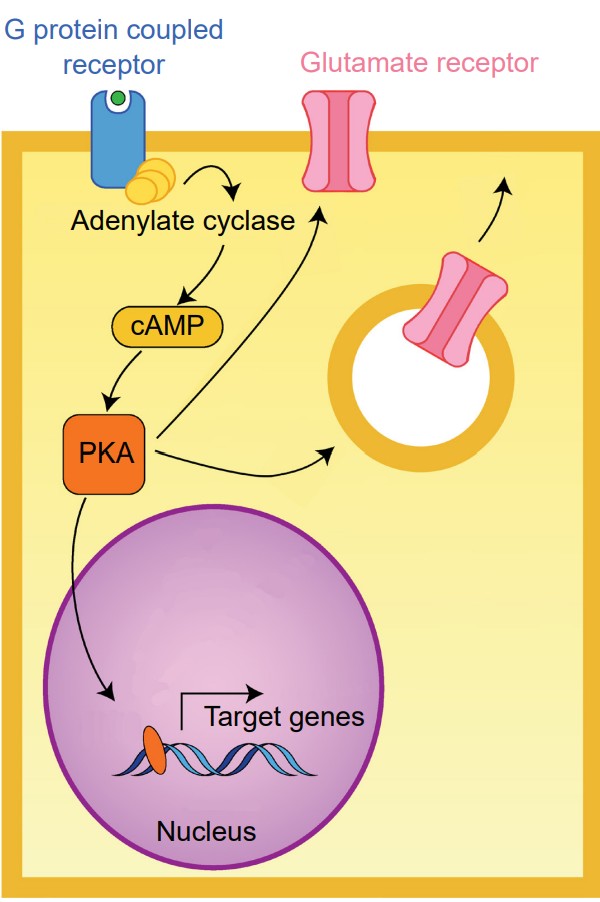
PKA is a kinase, an enzyme that is phosphorylates other proteins. The addition of a phosphate group onto a protein changes its properties dramatically. PKA phosphorylates protein targets that increase cell excitation. For example, one target of PKA activity is the intracellular side of certain glutamate receptors. Phosphorylation causes these receptors to stay open longer than normal when they are activated by a molecule of glutamate. This means that a single molecule of glutamate causes more excitation (passes more depolarizing current into the cell) in the presence of increased PKA activity.
Targets of PKA activity also include the intracellular store of glutamate receptors. When phosphorylated, these receptors are trafficked to the neuronal membrane. An increase of receptors at the postsynaptic side leads to increased excitatory neurotransmission over a period of minutes and hours, representing one form of plasticity.
An even longer-term action of PKA is its ability to change the transcription of various genes, which can trigger the synthesis of proteins. Some genes downstream of PKA activity include the structural protein actin, important for morphological changes in neuronal structure, or ion channels which change neuronal responses to neurotransmitter release.
Gαi. A GPCR that is coupled with Gαi causes a decrease in excitability. In many ways, Gαi proteins serve the opposite function as Gαs proteins – the “i” stands for inhibitory.
Whereas activation of Gαs increases the action of AC, Gαi proteins decrease AC activity. Therefore, Gαi activation decreases the intracellular concentration of cAMP, in turn decreasing PKA activity. Given the function of PKA as a kinase that increases cellular excitation as described above, decreased PKA activity inhibits cellular activity through multiple mechanisms, some of which include decreased current through glutamate receptors, decreased trafficking of glutamate receptors to the presynaptic neuronal membrane, and decreased transcription of certain genes.
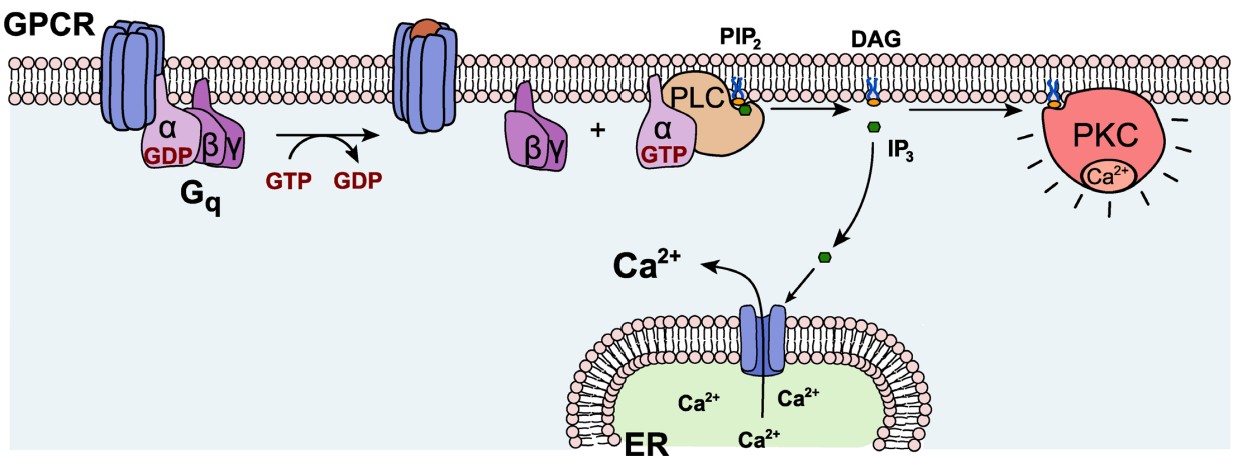
Gαq. Generally, Gαq is an excitatory G protein. Gαq uses a different signaling pathway compared to the PKA pathway that is downstream of Gαs or Gαi. Gαq protein activation leads to activity of the enzyme phospholipase C (PLC).
On a biomolecular level, PLC acts on the phospholipid membrane molecule phosphatidylinositol 4,5-bisphosphate (PIP2). PLC is a hydrolytic enzyme, and it breaks PIP2 into two separate second messenger molecules: the soluble inositol triphosphate (IP3) and the membrane-embedded diacylglycerol (DAG). One of the functions of IP3 is to liberate Ca2+ from intracellular stores, which elevates intracellular Ca2+ levels, depolarizing the cell and activating calcium-dependent processes, which are often excitatory. DAG activates protein kinase C (PKC), an enzyme with substrates that increase neurotransmitter release probability or decrease potassium channel conductance.
While the alpha subunits carry out a large part of GPCR-mediated changes in cellular excitation, the beta and gamma subunits also affect excitability. The beta and gamma subunits are bound together as a dimer, but they separate from the alpha subunit once the GPCR becomes activated. The beta-gamma complex can also function as a signaling molecule.
Compared to ionotropic receptors, metabotropic receptors affect neuron activity on a slower time scale, on the range of milliseconds to seconds, and possibly even longer depending on the downstream mechanisms that are activated.
Presynaptic receptors
In the discussion of receptors, it is common to think of receptors as being expressed at the dendrites, embedded within the postsynaptic membrane. However, not all receptors are located here. Some receptors are presynaptic, meaning they can be found at the axon terminal. Presynaptic receptors are often inhibitory and serve a self-regulatory function. Presynaptically-expressed receptors that respond to the same neurotransmitter that is released are called autoreceptors.
5.4 Neurotransmitters
As described previously, neurotransmitters are the substances that are released at chemical synapses, and they are the signaling molecules that allow neurons to communicate with one another. To date, scientists have identified more than 100 neurotransmitters. Here, we will describe six classical neurotransmitters, their receptors, and their actions. Additionally, three atypical neurotransmitters will be introduced.
One important note to keep in mind as you think about neurotransmitters: the effect that a neurotransmitter has on a cell depends on the receptor. In other words, a neurotransmitter molecule can either excite or inhibit a neuron depending on the composition of receptors that are present. For example, glutamate is excitatory at most synapses in the nervous system. Glutamate exerts excitation by activating ionotropic glutamate receptors, which are ligand-gated cation channels. However, at one particular synapse in the eye, glutamate activates a metabotropic glutamate receptor that causes cellular inhibition.
Glutamate
Glutamate (Glu) is the main excitatory neurotransmitter used by the nervous system. Glutamate is the same as the amino acid glutamic acid. There is more glutamate per volume of brain tissue than any other neurotransmitter. Glutamatergic neurons are identified by the presence of the vesicular glutamate transporter (vGluT).
Glutamate can activate both ionotropic and metabotropic receptors. Ionotropic glutamate receptors are all ligand-gated cation channels, which makes them excitatory since they allow Na+ to move into the cell. Ionotropic glutamate receptors are generally subdivided into three classes, named after exogenous chemicals that can activate the receptor. AMPA receptors are Na+ channels, but some also allow Ca2+ entry. NMDA receptors allow both Na+ and Ca2+ to pass across the membrane. When the cell is at rest, NMDA receptors also have a large magnesium ion in the pore that blocks ion movement through the channel. The third category of ionotropic glutamate receptors is called kainate receptors, which are similar to AMPA receptors.
The metabotropic glutamate receptors (mGluRs) signal using different G proteins. There are a total of 8 of these mGluRs, classified into three groups, called Group I, Group II, and Group III. Group I are excitatory GPCRs which signal via Gq, while Group II and Group III are inhibitory via the Gi signal transduction pathway.
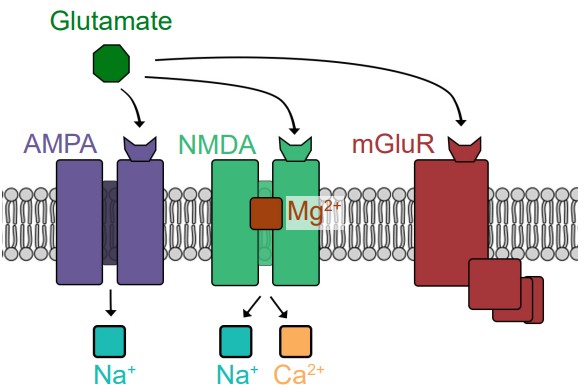
One theory proposes that excess signaling by glutamate can lead to neuronal death, a phenomenon called excitotoxicity. Of the various glutamate receptors, the NMDA receptor is most strongly implicated in contributing to excitotoxicity, since uncontrolled elevated levels of calcium can be deadly for neurons. Excitotoxicity is observed in a variety of disease states ranging from neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis, but also in injury such as concussion or stroke.
GABA + glycine
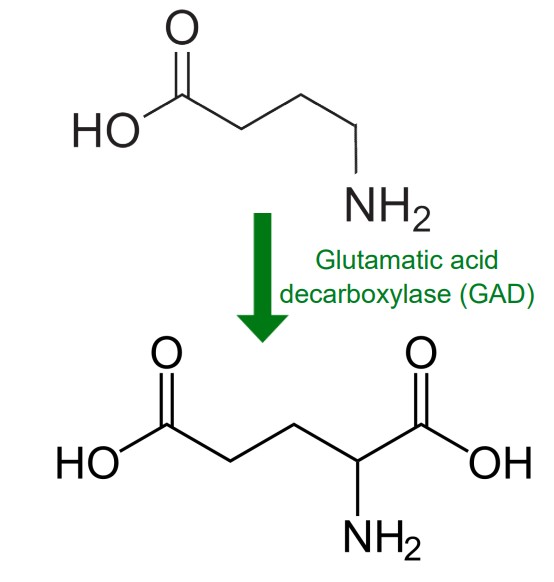
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the brain. According to one estimate, about 25% of neurons in the brain are GABA-ergic. Chemically speaking, GABA is remarkably similar to glutamate. In fact, GABA is synthesized from glutamate in a single step by the enzyme glutamic acid decarboxylase (GAD). GAD is often used as a biochemical marker for the presence of GABAergic neurons. Many interneurons use GABA as their chemical signaling molecule.
The main action of GABA as an inhibitory neurotransmitter is to activate one of three main classes of receptors, called A, B, and C. GABAA receptors are ligand-gated chloride channels, so activating these ion channels causes Cl- flux, which opposes the ability for the cell to reach action potential threshold. GABAB and GABAC receptors are both metabotropic receptors that inhibit neuronal activity through the action of the Gi protein.
A neurotransmitter that is similar to GABA is glycine. Another small amino acid, glycine is mostly used by neurons of the spinal cord and brain stem. Glycine is also inhibitory , and acts at glycine receptors, which are ligand-gated chloride channels.
Dopamine
Dopamine (DA) is a biogenic amine derived from the amino acid tyrosine through the action of several enzymes. One in particular, tyrosine hydroxylase (TH), is the main marker that is used for identifying dopamine-producing neurons. Unlike glutamate or GABA, dopamine-producing neurons are not widely abundant in the brain. Instead, there are generally only a few patches of neurons that produce dopamine, most of which are found in the midbrain. Two areas include the ventral tegmental area and the substantia nigra.
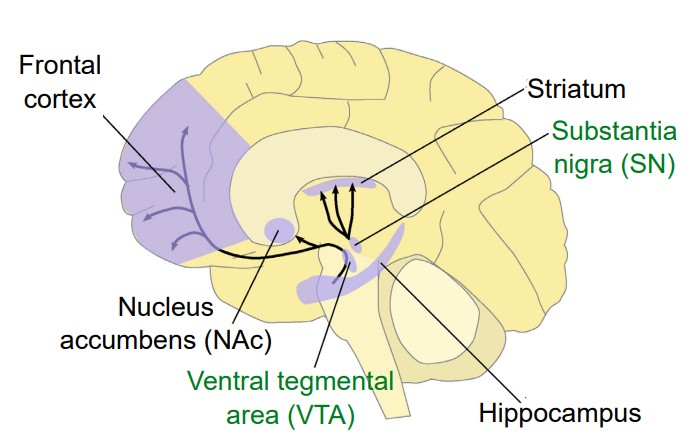
There are five classes of dopamine receptors, named D1 through D5. All of them are metabotropic receptors. D1 and D5 are generally excitatory receptors, while D2, D3, and D4 are inhibitory receptors.
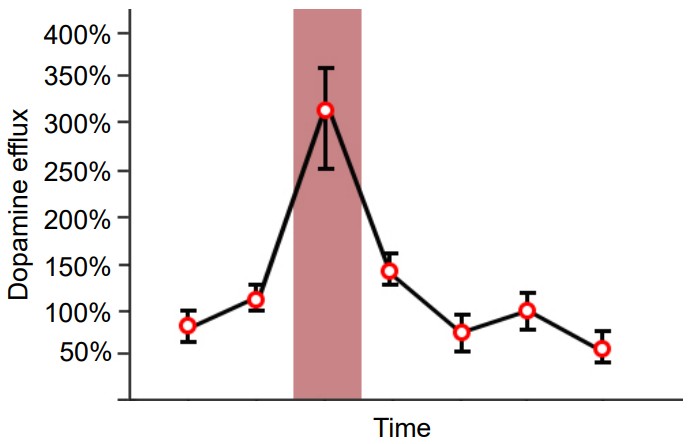
Since Roy Wise’s theory proposed in the 1960s, DA has been known in pop culture as the “pleasure neurotransmitter” because of its involvement in the processing of reward and motivation. For example, if we use microdialysis (a technique to measure the concentration of chemicals) in the nucleus accumbens, dopamine levels spike in response to all sorts of pleasurable or rewarding stimuli: food, water, sex, sugar, and exposure to drugs of abuse. However, we now know that dopamine is much more complex than once believed. One theory suggests that dopamine elevation serves as a “learning signal” that causes us to pay attention to salient stimuli in the environment.
DA is also needed for normal motor control. When dopamine-producing neurons in the substantia nigra pars compacta (SNpc) degenerate, as in Parkinson’s disease, a person develops the trademark symptoms: difficulties with motor control, resulting in a resting tremor, postural instability, and bradykinesia (slowness of movement). Reversing the dopamine deficiency by introducing an exogenous source of dopamine is our current gold standard of treatment for PD.
Serotonin
Serotonin (5-HT) is a neurotransmitter that is derived from the dietary amino acid tryptophan. The enzyme tryptophan hydroxylase is the first step of serotonin biosynthesis and is often used as a marker to identify serotonergic neurons. As with dopamine, there are only a few areas of the brain that synthesize serotonin, the major one being the Raphe nucleus in the brain stem.
Receptors for serotonin have a wide variety of actions. We have identified seven major families of 5-HT receptors, which are designated by the number and subclasses which are designated by a letter. For example, the 5-HT2A receptor is metabotropic and excitatory via Gq signaling, while the 5-HT5 receptor is inhibitory via Gi signaling. Most of them are metabotropic receptors; only the 5-HT3 receptor is ionotropic.
Serotonin is heavily implicated in the regulation of mood and complex behavioral conditions. One of our most effective strategies for treating depression is the administration of a drug such as fluoxetine, which acts as a selective-serotonin reuptake inhibitor (SSRI). Pharmacologically, fluoxetine increases synaptic levels of serotonin by preventing reuptake, and for some people, this has a moderate ability to reverse depression. Serotonin signaling is also a target for drugs that treat anxiety, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, and more.
Clinical correlation: Parkinson’s disease (PD) and L-DOPA-induced dyskinesia (LID)
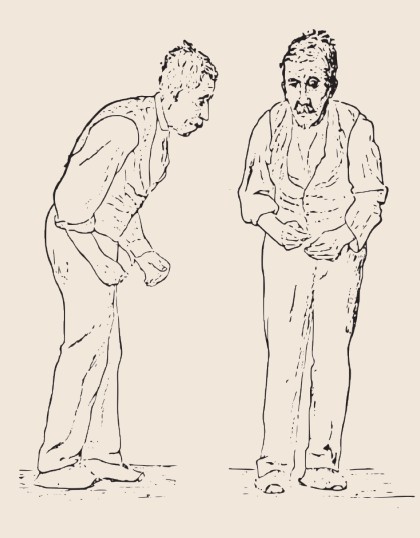 Parkinson’s disease is a debilitating neurodegenerative disorder that affects as many as 1% of all people aged 60 or older. Generally, PD is lethal within 16 years. By the time a patient presents to the clinic with motor dysfunction, they have already lost almost 60-80% of dopamine-producing neurons in this area!
Parkinson’s disease is a debilitating neurodegenerative disorder that affects as many as 1% of all people aged 60 or older. Generally, PD is lethal within 16 years. By the time a patient presents to the clinic with motor dysfunction, they have already lost almost 60-80% of dopamine-producing neurons in this area!
For decades, clinicians have been using the biochemical precursor to dopamine, L-DOPA, to treat the symptoms. However, after chronic exposure to L-DOPA, the drug becomes less effective and has a shorter duration of therapeutic action. Worse still, frequent treatment can lead patients to develop hyperkinesias, an abnormal excess of movements. This iatrogenic disorder is called L-DOPA induced dyskinesia (LID).
Biomedical engineers have developed a promising non-drug approach to treating PD called deep brain stimulation. A small stimulating device is surgically implanted into the subthalamic nucleus of the brain. When this brain area is stimulated, neural circuits are recruited which restores normal motor control.
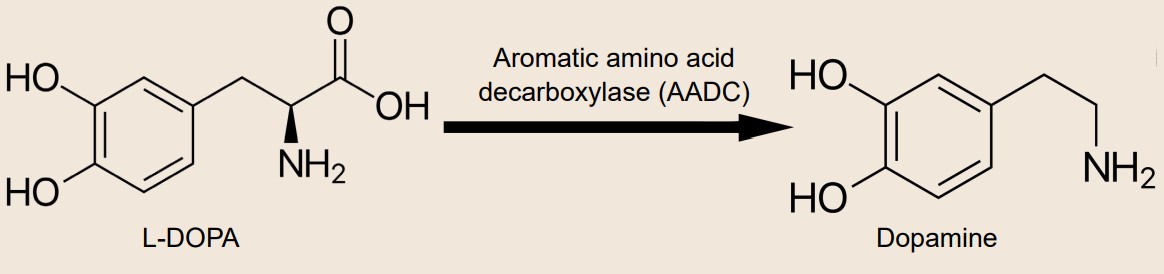
Acetylcholine
Acetylcholine (ACh) is a small molecule that is made by the enzyme choline acetyltransferase (ChAT), which chemically bonds a molecule of acetyl-CoA with a molecule of choline. The presence of ChAT in a neuron is used as a biochemical marker for neurons that produce acetylcholine.
ACh was the first neurotransmitter discovered and chemically isolated, a feat which earned two researchers the shared Nobel Prize in Physiology or Medicine in 1936. One of the two scientists, a German pharmacologist named Otto Loewi, stimulated the vagus nerve connected to an isolated frog heart, which caused the heart rate to slow down. When he put the surrounding solution on top of another heart, he observed that the second heart also slowed down, despite having no physical connection to the first heart. From this, he concluded that a chemical released by the vagus nerve is able to decrease heart rate. This chemical was first called Vagusstoff, the German word meaning Vagus substance. Today, we know it as acetylcholine.
ACh is able to act at ionotropic and metabotropic receptors, and activity at both receptor classes is essential for normal function. The ionotropic receptors of the nervous system are called nicotinic acetylcholine receptors (nAChRs) because they can be activated by nicotine in addition to acetylcholine. These ionotropic receptors are ligand-gated sodium channels and are therefore excitatory. On the other hand, the metabotropic receptors are called muscarinic acetylcholine receptors (mAChRs) since they are activated by the chemical muscarine found in some species of mushrooms. MAChRs can be coupled with either Gs or Gi , so they can be either excitatory or inhibitory.
ACh is the main neurotransmitter that the nervous system uses in order to communicate with the muscles at the neuromuscular junction (NMJ). Here, ACh is released by motor neurons, where it activates nicotinic acetylcholine receptors on muscle cells, causing them to constrict, or flex. On the other hand, muscarinic acetylcholine receptors are located in the heart, and their activation causes a decrease in heart rate (as Otto Loewi demonstrated with the isolated frog heart preparation).
In the central nervous system, ACh is involved in a wide variety of processes, including attention and learning. One of the first theories to explain the symptoms of Alzheimer’s disease looked at a loss of ACh-producing neurons in the basal forebrain that become more severe as the disease worsens. It has since been demonstrated that Alzheimer’s disease is more complex than this early hypothesis.
Norepinephrine
Norepinephrine (NE) is a neurotransmitter that is synthesized from a molecule of dopamine by the enzyme dopamine beta-hydroxylase. Norepinephrine-producing cells are localized in the pons of the brain stem, a structure called the locus coeruleus. The locus coeruleus is very small, but these neurons send projections widely throughout the brain.
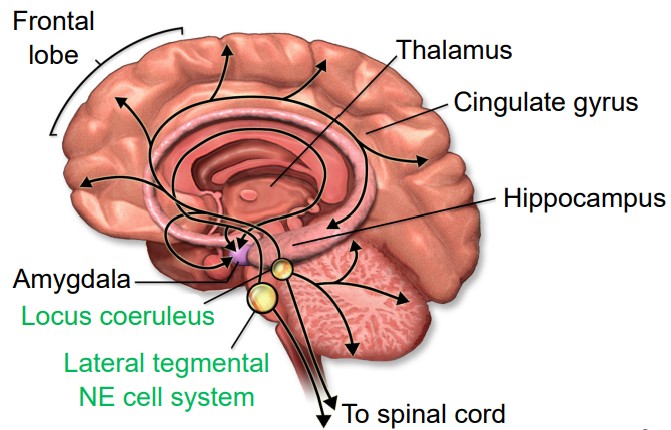
Outside of the brain, we think of norepinephrine as being responsible for triggering the sympathetic nervous system response of the body, the “fight-or-flight” reaction that prepares the body for physical activity in times of fear or acute stress. These norepinephrine-producing nerve cells reside in the sympathetic ganglia, a clump of nerve cells that run parallel to the spinal cord, one on each half of the body. These neurons project out towards the internal organs.
Receptors for NE are classified into two main categories, alpha or beta. There are subtypes within each category, giving us five major receptors for NE: alpha-1, alpha-2, beta-1, beta-2, and beta-3. All five of these receptors are metabotropic, and some are excitatory while others are inhibitory. Our internal organs express these noradrenergic receptors. Clinically, the “beta blockers” are a class of drugs that inhibit beta-adrenergic receptors; the resulting action is a decrease in blood pressure. Conversely, some beta-agonists are used as bronchodilators for asthma.
Norepinephrine also functions in the brain to modulate behaviors including alertness and attention.
Atypical neurotransmitters
Although we generally think of neurotransmitters as neurochemicals that function as described above, there are a few atypical neurotransmitters that don’t quite fit the mold of the other chemical signals.
Neuropeptides
Neuropeptides are a class of large signaling molecules that some neurons synthesize. Neuropeptides are different from the traditional neurotransmitters because of their chemical size. Monoamines like DA, NE, or 5-HT have a molecular weight around 150- 200, while one of the smaller neuropeptides, enkephalin, has a molecular weight of 570. One of the largest, dynorphin, has a molecular weight greater than 2,000. Because of their large size, neuropeptides have to be packaged in dense-core vesicles very close to the site of production near the nucleus rather than in clear vesicles right at the terminal.
Neuropeptides such as enkephalin and dynorphin are agonists at a class of receptors called the opioid receptors. These opioid receptors fall into four main types. The three classical opioid receptors are named using Greek letters: δ (delta), μ (mu), and κ (kappa), and the fourth class is the nociceptin receptor. All of these receptors are inhibitory metabotropic receptors which signal using the Gαi protein.
These receptors are expressed in several brain areas, but expression is particularly heavy in the periaqueductal gray, a midbrain area that functions to inhibit the sensation of pain. Drugs that activate the opioid receptors, like morphine, oxycontin, or fentanyl, are the most effective clinical treatments that we know of for acute pain. Unfortunately, these same drugs also represent a tremendous health risk, as opioid drugs can be lethal in overdose and have a high risk of substance use disorder.
Endocannabinoids (eCBs)
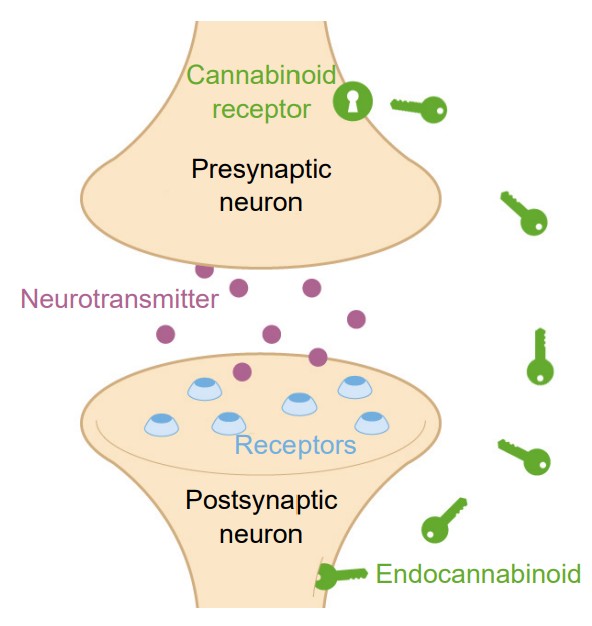
The eCBs are a class of lipidbased neurotransmitters. They are unusual neurochemicals in a few ways. Instead of sending information from the axon of one neuron to the dendrite of the next neuron (anterograde signaling), eCBs allow the postsynaptic dendritic component to communicate with the presynaptic axon terminal. Since they communicate information in the “opposite” direction of classic neurotransmitter signaling, eCBs are called retrograde signaling molecules. Secondly, eCBs are not packaged into vesicles and released by fusion processes. Instead, eCBs are synthesized de novo, meaning they get created right when they needed and used at that moment. The two most well-characterized eCBs in humans are called 2-AG and AEA. ECBs activate one of two receptors, CB1 and CB2. Both of them are inhibitory metabotropic receptors that couple with Galphai. Generally, CB1 receptors are found in the nervous system, while the CB2 receptors are found elsewhere in the body, such as in the immune system.
The eCB system is widely used by various systems in the body. It is estimated that eCB receptors are the most abundant GPCRs in the whole body.
These substances were named because they are endogenous chemicals that are functionally similar to compounds found in plants of the genus Cannabis. One reason cannabis is used is because of its ability to interact with our eCB receptors.
Nitric oxide
The nervous system is capable of signaling via the gas nitric oxide (NO). This gasotransmitter is not stored in vesicles but rather is synthesized as it is needed. NO is formed when the amino acid arginine is degraded by the enzyme NO synthase (NOS).
Because NO is a gas, it easily permeates across cell membranes. Therefore, the receptors for NO do not need to be transmembrane proteins expressed on the cell surface. Instead, the receptor for NO is an intracellular receptor called soluble guanylate cyclase (sGC). SGC works through a signaling pathway that is different from other metabotropic receptors so far described. SGC is linked with the signaling molecule cyclic GMP (cGMP), which activates protein kinase G. PKG can either be excitatory or inhibitory, depending on the intracellular components.
Chapter summary
Neurons communicate with one another in a variety of ways. Anatomically, neurons are separated by a small extracellular gap called the synapse. This synapse may directly connect the intracellular cytoplasm, as in an electrical synapse. Alternatively, the gap may be much larger, and chemicals that get released and diffuse across the synapse in order to signal to the following neuron. These chemicals, the neurotransmitters, are stored in vesicles, tiny spheres that are located in the presynaptic axon terminal. The release of these chemicals is very closely regulated, and neurons have several mechanisms that regulate vesicular fusion.
Following release, the neurotransmitters diffuse across the synapse and can bind to the active site on transmembrane proteins called receptors. Upon binding a molecule of neurotransmitter, these receptors physically change shape, resulting in ion flow across the membrane (ionotropic receptors) or a change in the activity of intracellular signaling molecules (metabotropic receptors). Binding of neurotransmitter changes the excitability of neurons.
We have so far identified more than 100 neurotransmitters. Many of them are small molecules that are packaged in vesicles, which then diffuse from the presynaptic neuron to the postsynaptic neuron, such as acetylcholine, glutamate, or GABA. However, there are some atypical neurotransmitters such as neuropeptides, endocannabinoids, and nitric oxide that have different methods of communication.
Image Credits
5.1 https://upload.wikimedia.org/wikipedia/commons/7/78/Gap_cell_junction_keys.svg modified by Austin Lim
5.2 https://upload.wikimedia.org/wikipedia/commons/e/e4/A_skybridge_at_IUPUI_campus.jpg
5.3 https://upload.wikimedia.org/wikipedia/commons/e/ed/Charcot-marie-tooth_foot.jpg
5.4 (Left) https://upload.wikimedia.org/wikipedia/commons/4/4c/Synapse_diag1.svg modified by Austin Lim
(Right) https://commons.wikimedia.org/wiki/File:Synapse_diag4.png#file modified by Austin Lim
5.5 Tao C-L, Liu Y-T, Zhou ZH, Lau P-M and Bi G-Q (2018) Accumulation of Dense Core Vesicles in Hippocampal Synapses
Following Chronic Inactivity. Front. Neuroanat. 12:48. doi: 10.3389/fnana.2018.00048
5.8 https://upload.wikimedia.org/wikipedia/commons/2/28/Opening_of_a_Fusion_Pore_during_Exocytosis.png modified by
Austin Lim
5.9 https://upload.wikimedia.org/wikipedia/commons/1/1e/Botulinum_Toxin_Mechanism.png modified by Austin Lim
5.10 https://upload.wikimedia.org/wikipedia/commons/0/0c/0310_Exocytosis_cleaned.png modified by Austin Lim
5.11 https://upload.wikimedia.org/wikipedia/commons/e/e7/1226_Receptor_Types.jpg modified by Austin Lim
5.12 https://upload.wikimedia.org/wikipedia/commons/6/6d/GPCR-Zyklus.png
5.13 https://upload.wikimedia.org/wikipedia/commons/8/8f/CREB_cAMP_neuron_pathway.svg modified by Austin Lim
5.14 https://upload.wikimedia.org/wikipedia/commons/3/31/Activation_protein_kinase_C.svg modified by Austin Lim
5.17 https://upload.wikimedia.org/wikipedia/commons/d/de/Dopamine_pathways.svg modified by Austin Lim
5.18 Data from K.N. Segovia, M. Correa, J.D. Salamone, Slow phasic changes in nucleus accumbens dopamine release
during fixed ratio acquisition: a microdialysis study, Neuroscience, Volume 196, 2011, Pages 178-188, ISSN 0306-4522,
https://doi.org/10.1016/j.neuroscience.2011.07.078. Reprinted with permission 2/15/2020
5.19 https://upload.wikimedia.org/wikipedia/commons/4/44/Sir_William_Richard_Gowers_Parkinson_Disease_
sketch_1886.svg
5.20 https://upload.wikimedia.org/wikipedia/commons/a/aa/Electron_micrograph_of_neuromuscular_junction_%28crosssection%29.jpg
5.21 https://upload.wikimedia.org/wikipedia/commons/4/48/Norepinephrine_Part_1.png modified by Austin Lim
5.23 https://upload.wikimedia.org/wikipedia/commons/2/27/Sistema_endocannabinoide.png modified by Austin Lim
sensitive to electrical charge
some molecules are able to travel across the membrane easily, other molecules have an intermediate ability to cross, and other molecules are completely incapable of passing.
huge protein complexes that span the entirety of the membrane, with an outer side and an inner side
a “tunnel” that allows molecules and ions to pass across the cell membrane
Ligand-gated ion channels
the electrical forces acting on charged molecules, “pulling” opposite charges together while also “pushing” like charges away from one another
the natural process by which a high concentration of a substance, given enough time, will eventually diffuse to a lower concentration and settle evenly over the space.
two forces acting on an ion oppose one another perfectly
the exact membrane voltage value for an ion
an equation to predict the direction that ions will move when an ion channel opens given various conditions
equilibrium potential is sometimes referred to as reversal potential
equilibrium potential is also called the Nernst potential to honor Walther Nernst
an equation to quickly calculate the equilibrium potential
combines the Nernst potentials of three relevant ions (Na+, K+, and Cl- ) into a single equation that, when evaluated, gives us the value of the membrane potential
the ability for an ion to cross the membrane through ion channels
The electrical output of many neurons is an all-or-nothing response called an action potential.
sub-threshold changes in membrane voltage
when membrane potential goes from negative to a more positive potential
when membrane potential changed from positive to a more negative potential
the value that membrane potential needs to reach to initiate action potential
small deviations in membrane voltage
postsynaptic potentials
small depolarizations caused by the release of excitatory neurotransmitters
small hyperpolarizations caused by the release of inhibitory neurotransmitters
two small EPSPs from two adjacent inputs are triggered
multiple EPSPs from the same input occur close together in time
a dysregulation in somatosensory systems that cause people to experience pain even in the absence of injurious stimuli
the moments following the beginning of an action potential where a second action potential cannot be fired
a deadly voltage-gated sodium channel inhibitor
tetrodotoxin
An efferent signaling pathway that signals the diaphragm to move up and down
the speed at which an action potential travels down the length of the axon
The spaces of exposed axon between each section of myelin
a specialized physical channel between two connected cells that allows for the passage of cytoplasm. also called hemichannel
a specialized physical channel between two connected cells that allows for the passage of cytoplasm. also called connexon
transmembrane proteins that make up the hemichannel
the structure that connects neurons electrically
the space between a motor neuron and muscle tissue
short for neuromuscular junction
a technique using a beam of electrons aimed at a sample in a vacuum, and the reflection of those electrons can be collected and detected with a computer
vesicular transport which move GABA
vesicular transporter which move glutamate
vesicular transporter which move acetylcholine
vesicular transporter which move monoamines such as dopamine and serotonin
membrane-embedded proteins that utilize the molecular energy contained in ATP to concentrate H+ ions in the intravesicular space
short for vesicular ATP-ase
membrane proteins that transport molecules in opposite directions
v-SNARE protein thats involved during synaptic release
v-SNARE protein thats involved during synaptic release
t-SNARE protein that functions during vesicular fusion
short for synaptosomal nerve-associated protein 25, a t-SNARE protein that functions during vesicular fusion
large protein complexes that normally remain closed, but when the surrounding neuronal membrane becomes depolarized, they physically change conformation and open up, allowing ions to move across the cell membrane.
short for voltage-gated calcium channels
a molecular structure formed when the v-SNAREs and the t-SNAREs interact with one another in the presence of Ca2+
The specific series of amino acid residues on the extracellular surface of a receptor where neurotransmitter molecules bind
another term for the active site
Chemicals that bind to the active site of ionotropic receptors
another term for ionotropic receptors
proteins which induce changes in neuronal excitability through the action of second messenger signaling molecules
another term for metabotropic receptors
short for seven-transmembrane receptors
another term for metabotropic receptors
short for G protein-coupled receptors
chemically similar to ATP and can function as a source of energy
guanosine triphosphate thats been broken down
an enzyme that creates a second messenger, a molecule called cyclic AMP
a messenger that, when levels are elevated, activates an enzyme called protein kinase A
a kinase, an enzyme that is phosphorylates other proteins
a hydrolytic enzyme that breaks phospholipid membrane molecule phosphatidylinositol 4,5-bisphosphate (PIP2) into two molecules: inositol triphosphate (IP3) and diacylglycerol (DAG)
Presynaptically-expressed receptors that respond to the same neurotransmitter that is released
vesicular transporter which move glutamate
excess signaling by glutamate which leads to neuronal death
often used as a biochemical marker for the presence of GABAergic neurons
small amino acid mostly used by neurons of the spinal cord and brain stem. Glycine is also inhibitory, and acts at glycine receptors, which are ligand-gated chloride channels.
the main marker that is used for identifying dopamine-producing neurons
area in the midbrain that produces dopamine
area in the midbrain that produces dopamine
an enzyme that acts as a marker to identify serotonergic neurons
an area in the brain stem that produces serotonin
increases synaptic levels of serotonin by preventing reuptake
an abnormal excess of movements
disorder where hyperkinesias is developed from frequent L-DOPA treatment
a non-drug approach to Parkinson's disease treatment where a stimulating device is implanted into the subthalamic nucleus of the brain
a biochemical marker for neurons that produce acetylcholine
ionotropic receptors of the nervous system
the metabotropic receptors of the nervous system
an enzyme that synthesizes norepinephrine from a molecule of dopamine
inhibitory metabotropic receptors which signal using the Gαi protein
an intracellular receptor for nitric oxide