Psychopathology: Anxiety, Trauma, and the Schizophrenia Spectrum
Video – Author/Source: Ted Talks; What is schizophrenia? – Anees Bahji
Discover what we know— and don’t know— about the symptoms, causes, and treatments of schizophrenia. — Schizophrenia was first identified more than a century ago, but we still don’t know its exact causes. It remains one of the most misunderstood and stigmatized illnesses today. So what do we actually know about its symptoms, causes, and treatments? Anees Bahji investigates.
Reading – Anxiety and Related Disorders – Anxiety and Related Disorders | Noba (nobaproject.com)
Reading – Bremner & Wittbrodt – 2020 – Int Rev Neurobiol – Stress, the brain, and trauma spectrum disorders
Reflection Reading #6 – Pierce & Black, 2023 – Trauma Violence Abuse – The neurophysiology behind trauma-focused therapy modalities used to treat post-traumatic stress disorder across the life course: A systematic review
Resource – https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596
Chapter 16: Diseases of the Brain
The brain can be thought of as a well-oiled machine made up of hundreds of billions of moving parts, all cooperating in tandem for healthy behavior and function. There is redundancy in the way these parts are organized, allowing for occasional mishaps without any significant loss of function. But when these parts interact in unusual or atypical ways, a person may develop some psychiatric disorder. The conditions described in this section likely involve dysregulations in molecules, cells, or circuits, and are therefore complex.

As far as we know, the likelihood of developing these conditions is not exclusively determined by either genes or influence from the environment. Instead, there is probably some influence from both. That is to say, none of these conditions are 100% penetrant; none of them are dictated exclusively by genetics. Having two parents or an identical twin with the condition may indicate an elevated baseline risk over the population at large, but it is not a guarantee that the disease will manifest. Environmental triggers and other exposures may lead to a sudden onset of the condition; on the other hand, certain factors in the environment may be protective against these diseases.

One of the major challenges with understanding these brain diseases is related to the difficulty of making an accurate diagnosis – as almost everything in biology exists on a spectrum, so do these brain disorders. The symptoms of these disorders frequently overlap, adding another layer of complexity. To help establish a diagnosis, the American Psychiatric Association (APA) has put together a series of criteria for psychiatrists to diagnose these complex conditions. The guidelines are compiled in a book called the Diagnostic and Statistical Manual of Mental Disorders. They are currently on the fifth revision of the text, referred to as the DSM-5. It’s an imperfect set of criteria, but it is a start towards understanding these remarkably complicated conditions.
Many of the treatments we currently have for neuropsychiatric disorders aren’t always effective. Our ability to treat these conditions depends on our understanding of the disease. The better we understand the causes of these conditions, the wider variety of new therapies we can test. Therapeutic strategies for these conditions are often first tested using cells and non-human animal models, but these have shortcomings. Most of the time, animal models of disease incompletely mimic the symptoms of the disease. Animal research concerns itself with three different forms of validity.
1. Face validity. If an animal model of a disease looks similar to the human condition, whether behaviorally or in physical appearance, we say that the model has good face validity. The animal exhibits the same set of symptoms that you would see in a human affected by the same condition, such as a rat model of post-traumatic stress disorder (PTSD) where the rat is exposed to a predator. Future exposure to predator-associated cues causes the rat to exhibit anxiety and avoidance, which are symptoms of human PTSD. In this case, the model has good face validity.
2. Construct validity. Sometimes, an animal model of a disease starts with the same pathological changes in the brain that are observed in human patients. We say that these models have good construct validity. The risk of developing Huntington’s disease (Chapter 10) in humans is associated with the number of poly-glutamine repeats in the Huntingtin protein. Developing a genetically modified animal model that has several poly-glutamine repeats is an example of a model with good construct validity. Because humans and non-humans are very different animals, having the same origin of disease does not always produce the disease symptoms.
3. Predictive validity. An animal model has good predictive validity if the animal model can be used to predict whether a therapy would be effective in treating humans with that same condition. For example, if there were a genetic mouse model that showed symptoms of depression, and an experimental antidepressant reverses depression in both the mouse and humans with depression, the model would be said to have good predictive validity.

Unfortunately, we don’t have any animal models that reproduce the symptoms of the most complex human conditions – it is almost impossible to create a mouse model of dissociative identity disorder or dyslexia, and even if we could, scientists would struggle to quantify the behaviors that we use as diagnostic criteria, which are too subtle to be observed or quantified in non-humans. And for the animal models that we do have, they are often imperfect or incomplete, modeling only some of the deficits seen in humans. Furthermore, most human diseases have many symptoms, and only a few can be assessed with behavioral tests. We can only study disorders of the brain that have a clearly and easily quantifiable behavioral component.
16.1 Schizophrenia (SZ)
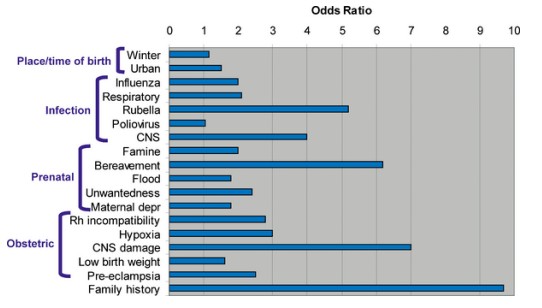
Schizophrenia is a psychiatric condition affecting just under 1% of people. SZ affects men slightly more often than women and affects people of all races. There is a strong association between low socioeconomic status and the risk of developing schizophrenia, indicating that stresses such as neonatal nutritional deficiency or food insecurity may be risk factors. Other risk factors that contribute to increased SZ risk include prenatal drug exposure, heavy drug use during early adolescence, and childhood adversity.

A diagnosis is generally made while a person is in their late adolescent years through their thirties. During this phase of life, the brain is still undergoing subtle maturation processes, which may account for why a person is more vulnerable in these years. After this age, the risk of developing SZ decreases significantly. Also, the later in life that SZ symptoms appear, the better the health outcomes are.
It is worth noting that people with SZ have the neurotypical range of intelligence, with the occasional outliers: John Nash, the real-life Nobel prize-winning economist depicted in the movie A Beautiful Mind, was first diagnosed with SZ in 1959.
Symptoms of schizophrenia
The symptoms of SZ can be roughly classified into two categories, positive symptoms and negative symptoms. These phrases are used to describe whether there is an excess of some function (positive symptoms) or a deficit of a function (negative symptoms). The symptoms do not appear uniformly across patients, so not all patients develop every symptom.

The most well-known positive symptom of schizophrenia is hallucinations, perceiving something that is not there (as opposed to illusions, which are misinterpretations of things that are there). Usually, patients experience auditory hallucinations, but they more rarely have visual hallucinations. The voices that these people hear may be consistent or can change over time. Interestingly, the nature of these hallucinations is influenced by society. In cultures with strong ancestor reverence, they may hear the voices of their grandparents, whereas people in religious cultures may hear the voices of deities.

Relatedly, people with SZ may experience a variety of delusions, untrue beliefs that cannot be changed despite overwhelming evidence. The delusions can come and go spontaneously. Delusions exist in many forms. A paranoid delusion is when a person believes that they are being spied on, maybe by the government or by aliens. A persecutory delusion is a persistent thought that the world is out to get them or to do them harm. Delusions of grandeur are when a person has a tremendously high sense of selfesteem, believing that they are royalty or are the reincarnation of God.
The negative symptoms of SZ may include deficits in expression. One common symptom is a flat affect, where a patient does not show or express emotion in situations where you would expect to see them. A related negative symptom is alogia, a decrease in the use of language. People with alogia often use vague language that is lacking in content or repetitive.
Negative symptoms also include deficits in motivation or interest. Two closely-related negative symptoms include anhedonia, a loss of a sensation of pleasure and the inability to expect upcoming pleasure. Furthermore, patients with SZ may also exhibit avolition, a decrease in goal-directed activity, which can cause a person to stop seeing their friends and cease displaying interest in social gatherings, leading to worsened interpersonal relationships.

Other negative symptoms that can present in SZ are a variety of motor disturbances. Basal ganglia and cerebellar structural deficits are found in SZ, two brain structures involved in motor control (see chapter 10), which may explain why deficits are observed. One motor difficulty is catatonia, where a person can hold their body in a highly unusual position for a prolonged period of time. They may also display stereotypy, a series of repetitive, purposeless behaviors, such as the persistent rocking of the body or self-caressing.
Negative symptoms may also manifest as a deficit of a patient’s cognitive abilities, particularly shortcomings in episodic memory (Chapter 13). They may also present with difficulty in performing attention-related behavioral tasks.
Potential causes of schizophrenia
In a healthy person, dopamine is important for motor control and motivation, two behaviors that are changed in patients with SZ. Therefore, scientists have suggested that abnormal dopamine signaling may be an underlying root cause. While the dopamine hypothesis is one of the earliest theories of SZ, modern genetics studies have shown that polymorphisms in the dopamine D2 receptor are risk factors.
Atypical cortical neuron network development is also likely to be present in SZ. In the healthy brain, networks of cortical neurons produce cyclic patterns of activity in the 40 Hz range, a pattern called a gamma oscillation. These gamma oscillations result from a combination of excitatory and inhibitory neurons. In SZ, there is a decrease in the density of dendritic spines on the excitatory neurons with a simultaneous decrease in GABA-ergic signaling, which leads to unpredictable gamma oscillations.
Animal models of schizophrenia
One animal model for SZ is based on the hypothesis that excess dopamine leads to the disorder. Introducing high doses of the drug amphetamine, which increases dopaminergic signaling, induces a temporary schizophrenic-like state in non-human animals. The hyperdopaminergic model of SZ produces cognitive deficits with no changes in memory or other negative symptoms. Alternatively, administration of NMDA glutamate receptor antagonists, like ketamine or PCP, is also used as a behavioral model of SZ.
Other non-human models of SZ are neurodevelopmental models. In these models, a pregnant dam is exposed to the compound MAM, which causes the newborns to develop atypically and display behavioral deficits similar to SZ. Inducing an unusually strong immune response in the pregnant mother can also cause atypical development in utero, which causes the animals to experience behavioral deficits after birth.
The biggest limiting factor to developing an animal model is that many symptoms in human SZ, like paranoid delusions or auditory hallucinations, are impossible to detect and quantify in non-humans. The PCP model can cause changes in rodent social behaviors, but it is hard to tell if this model causes any of the positive symptoms that you might see in a patient with SZ. Despite the limitations of these non-human models of SZ, they have been helpful in testing the therapeutic efficacy of anti-schizophrenia drugs.
Treatments of SZ
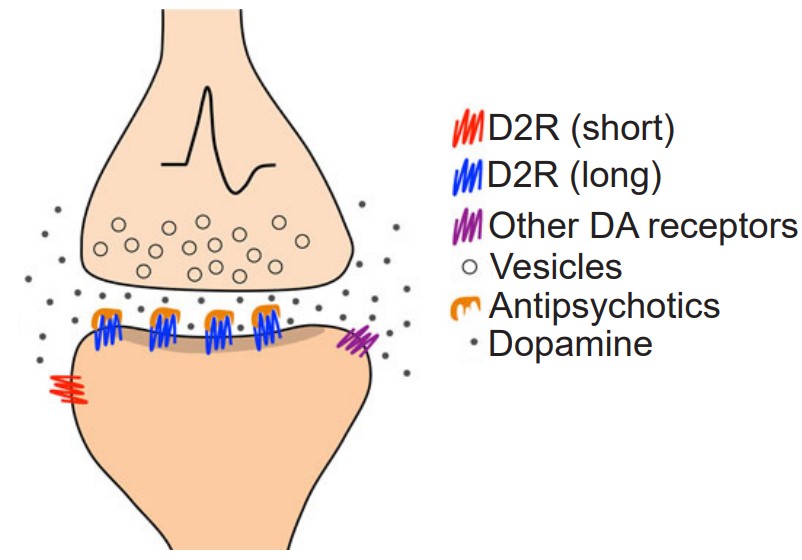
The dopamine theory of SZ has led to a few novel therapeutic strategies, especially in the context of the dopamine D2 receptor. D2 antagonists decrease hallucinations and delusions in some patients with SZ, and the effectiveness of the antagonist is correlated with the ability of that drug to block the D2 receptor. Clozapine, an atypical antipsychotic that functions as a dopamine receptor antagonist, can decrease SZ symptoms as well.
Unfortunately, pharmacological therapies are not always effective in humans. Around a third of patients discontinue their treatment regimen, and around a fifth of them report adverse side effects such as extrapyramidal motor symptoms, sedation, and weight gain.
A potential new therapy is based on transcranial magnetic stimulation (Chapter 6). Some evidence suggests that targeted activation of the cortex can decrease the severity of auditory hallucinations. There may also be some mild improvements in the negative symptoms.
Approximately 65% of North Americans with SZ are smokers, compared to 25% in the population at large. If they are smoking as self-medication to activate dopamine- or acetylcholine-sensitive networks in the brain, this observation may lead to a new therapeutic strategy. Alternatively, they may smoke to get pleasure, which acts to reverse the anhedonia that is one of the main symptoms of the disease.
Image Credits
16.1 https://pixabay.com/photos/children-twins-girls-young-652270/
16.2 https://upload.wikimedia.org/wikipedia/commons/4/4b/Dsm-5-released-big-changes-dsm5.jpg
16.3 https://pixabay.com/photos/cat-animal-cute-pet-room-play-3162458/
16.4 https://upload.wikimedia.org/wikipedia/commons/a/a9/John_Forbes_Nash%2C_Jr._by_Peter_Badge.jpg
16.5 https://upload.wikimedia.org/wikipedia/commons/a/a6/Schizophrenia_image.jpg
16.6 https://pixabay.com/photos/hiding-boy-girl-child-young-box-1209131/
16.7 https://wellcomecollection.org/works/wv5n6jjx
16.8 https://upload.wikimedia.org/wikipedia/commons/7/7f/Schizophrenia_risk_factors-PLOS.png
16.9 Amato D, Kruyer A, Samaha A-N and Heinz A (2019) Hypofunctional Dopamine Uptake and Antipsychotic TreatmentResistant Schizophrenia. Front. Psychiatry 10:314. doi: 10.3389/fpsyt.2019.00314
degree to which a particular gene or set of genes will manifest a specific trait or condition
the handbook created by the American Psychiatric Association used by health care professionals in the United States and much of the world as the authoritative guide to the diagnosis of mental disorders
The version of the Diagnostic and Statistical Manual of Mental Disorders that is currently being used
one of two categories of schizophrenia symptoms; describes an excess of some function
one of two categories of schizophrenia symptoms; describes a deficit of some function
perceiving something that is not there; most well-known positive symptom of schizophrenia
untrue beliefs that cannot be changed despite overwhelming evidence
symptom of schizophrenia where a patient does not show or express emotion in situations where you would expect to see them
negative symptom of schizophrenia; a decrease in the use of language
a loss of a sensation of pleasure and the inability to expect upcoming pleasure
a decrease in goal-directed activity
motor difficulty associated with schizophrenia where someone can hold their body in a highly unusual position for a prolonged period of time
a series of repetitive, purposeless behaviors
suggestion that abnormal dopamine signaling may be an underlying root cause of schizophrenia


